Question: > Question 3 1 pts Calculate the initial molar concentration of KNO, for Trial 3 given the following contents of the reaction mixture. All 6
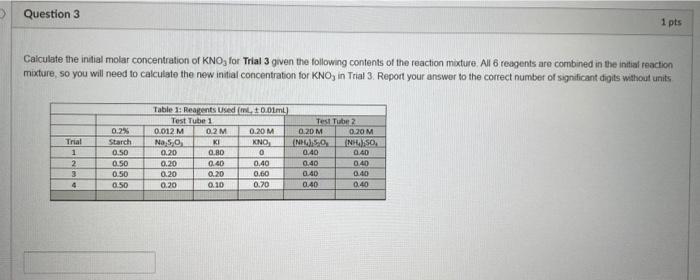
> Question 3 1 pts Calculate the initial molar concentration of KNO, for Trial 3 given the following contents of the reaction mixture. All 6 reagents are combined in the initial reaction mixture, so you will need to calculate the new initial concentration for KNO, in Trial 3. Report your answer to the correct number of significant digits without units Trial 1 2 3 4 0.2% Starch 0.50 0.50 0.50 0.50 Table 1: Reagents Used 0.0 mL) Test Tube 1 Test Tube 0.012 M 02 M 0.20 M 0.20 M 0.20M NOSO KI KNO, (NP.50 (NISO 0.20 0.BD 0 0.40 0.40 0,20 0.40 0.40 0.40 0.40 0.20 0.20 0.60 0.40 0.40 0.20 0.10 0.70 0.40 0.40
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


