Question: Question # 3 2 5 Marks Water - soluble polymer PVA ( Polyvinyl Alcohol ) is degraded in a photochemical reactor where the polymer degradation
Question #
Marks
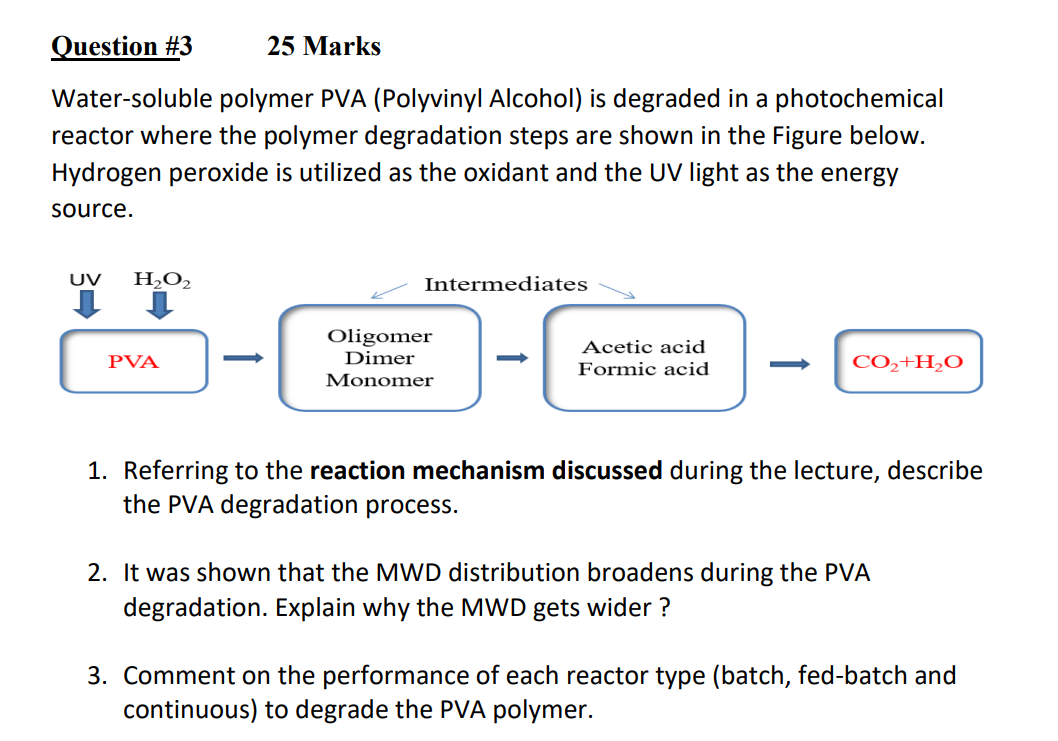
Watersoluble polymer PVA Polyvinyl Alcohol is degraded in a photochemical
reactor where the polymer degradation steps are shown in the Figure below.
Hydrogen peroxide is utilized as the oxidant and the UV light as the energy
source.
Referring to the reaction mechanism discussed during the lecture, describe
the PVA degradation process.
It was shown that the MWD distribution broadens during the PVA
degradation. Explain why the MWD gets wider
Comment on the performance of each reactor type batch fedbatch and
continuous to degrade the PVA polymer.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


