Question: Question 3 2 pts The half life for the decomposition of a particular compound is 20.7 days. What is the rate constant for this first-order
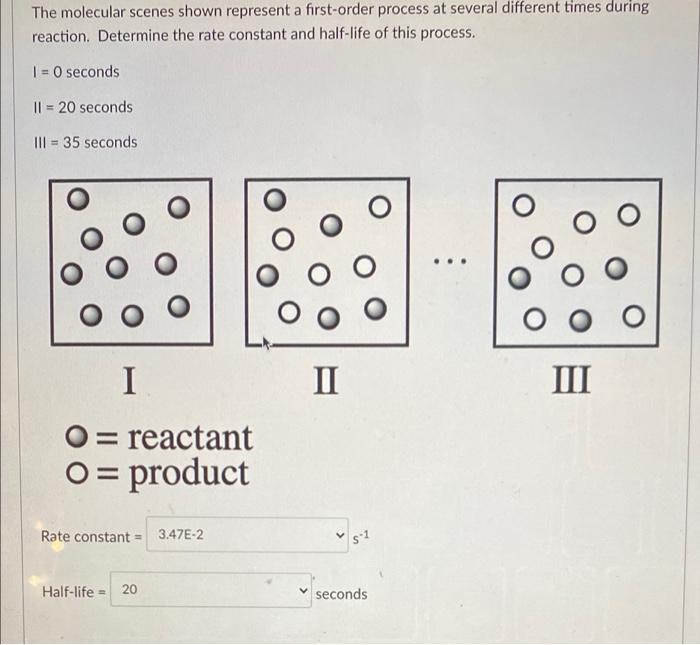
Question 3 2 pts The half life for the decomposition of a particular compound is 20.7 days. What is the rate constant for this first-order process in units of day 1? The molecular scenes shown represent a first-order process at several different times during reaction. Determine the rate constant and half-life of this process. 1 = 0 seconds 11 = 20 seconds III = 35 seconds ... I II III O= reactant O= product Rate constant = 3.47E-2 $2 Half-life = 20 seconds sece
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


