Question: QUESTION 3 (20 MARKS) a. For the cell notation below; Few Fe y Ag Ag i. Write the half equation at anode and cathode ii.
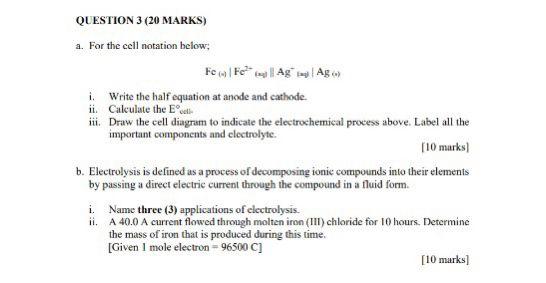
QUESTION 3 (20 MARKS) a. For the cell notation below; Few Fe y Ag Ag i. Write the half equation at anode and cathode ii. Calculate the call iii. Draw the cell diagram to indicate the electrochemical process above Label all the important components and electrolyte. [10 marks b. Electrolysis is defined as a process of decomposing ionic compounds into their elements by passing a direct electric current through the compound in a fluid form i. Name three (3) applications of electrolysis. ii. A 40.0 A current flowed through molten iron (III) chloride for 10 hours. Determine the mass of iron that is produced during this time. [Given 1 mole electron - 96500 C] [10 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


