Question: Question 3 (20 points): 1.00 0.90 0.80 0.70 0.60 I Xe, 0.50 0.40 0.30 0.20 0.10 0.00 200 300 400 500 700 800 900 1000
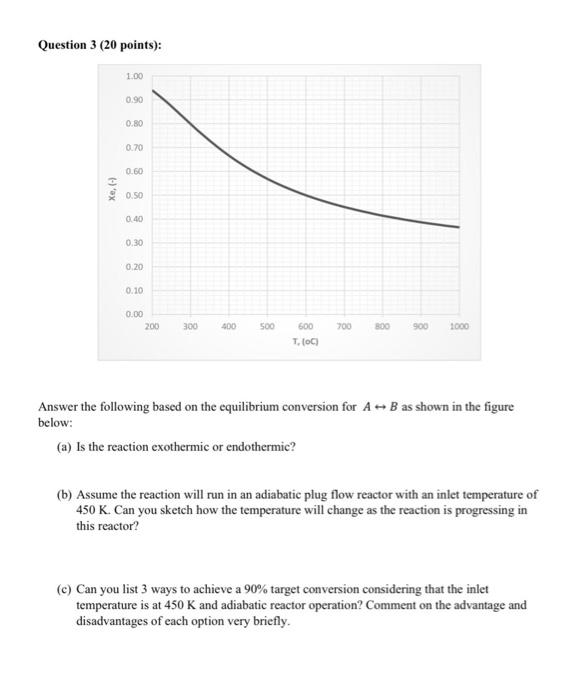
Question 3 (20 points): 1.00 0.90 0.80 0.70 0.60 I Xe, 0.50 0.40 0.30 0.20 0.10 0.00 200 300 400 500 700 800 900 1000 600 T. (C) Answer the following based on the equilibrium conversion for A - B as shown in the figure below: (a) Is the reaction exothermic or endothermic? (b) Assume the reaction will run in an adiabatic plug flow reactor with an inlet temperature of 450 K. Can you sketch how the temperature will change as the reaction is progressing in this reactor? (c) Can you list 3 ways to achieve a 90% target conversion considering that the inlet temperature is at 450 K and adiabatic reactor operation? Comment on the advantage and disadvantages of each option very briefly
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


