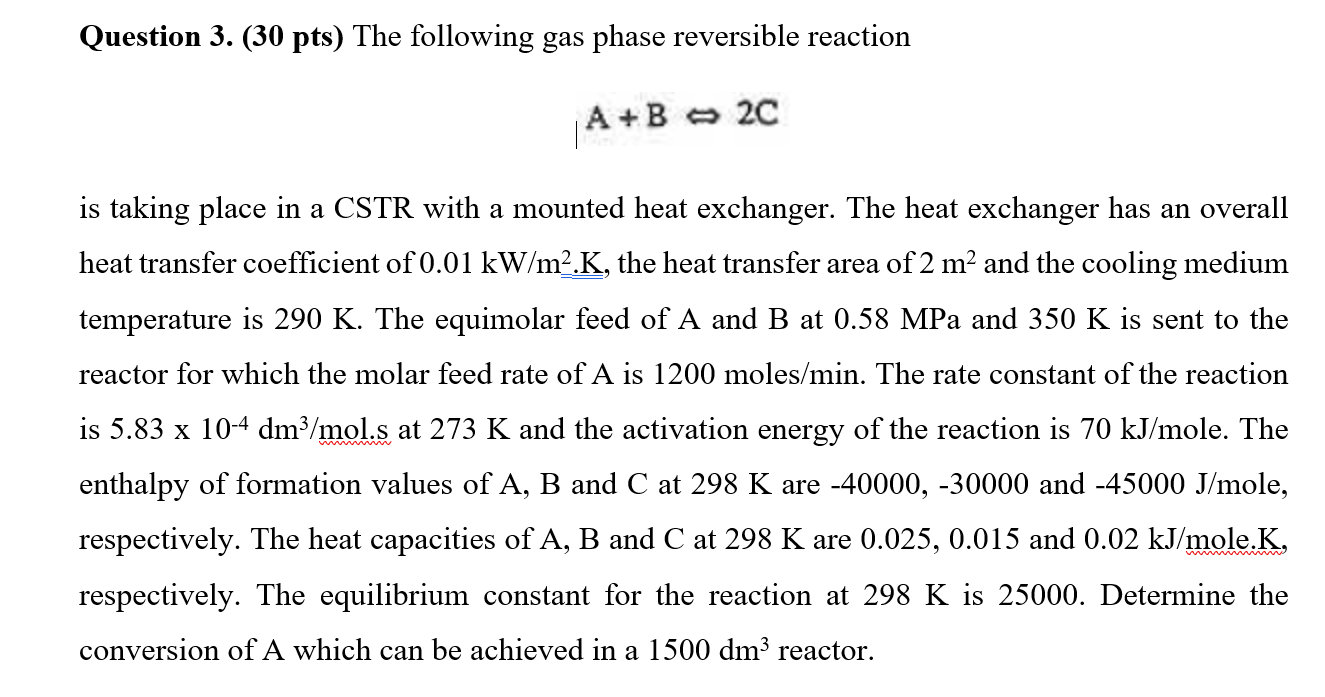
Question: Question 3 . ( 3 0 pts ) The following gas phase reversible reaction A + B = > 2 c is taking place in
Question pts The following gas phase reversible reaction
ABc
is taking place in a CSTR with a mounted heat exchanger. The heat exchanger has an overall
heat transfer coefficient of the heat transfer area of and the cooling medium
temperature is The equimolar feed of A and at MPa and is sent to the
reactor for which the molar feed rate of is mole The rate constant of the reaction
is at and the activation energy of the reaction is ole. The
enthalpy of formation values of A B and C at are and ole,
respectively. The heat capacities of A B and C at are and ole.
respectively. The equilibrium constant for the reaction at is Determine the
conversion of A which can be achieved in a reactor.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


