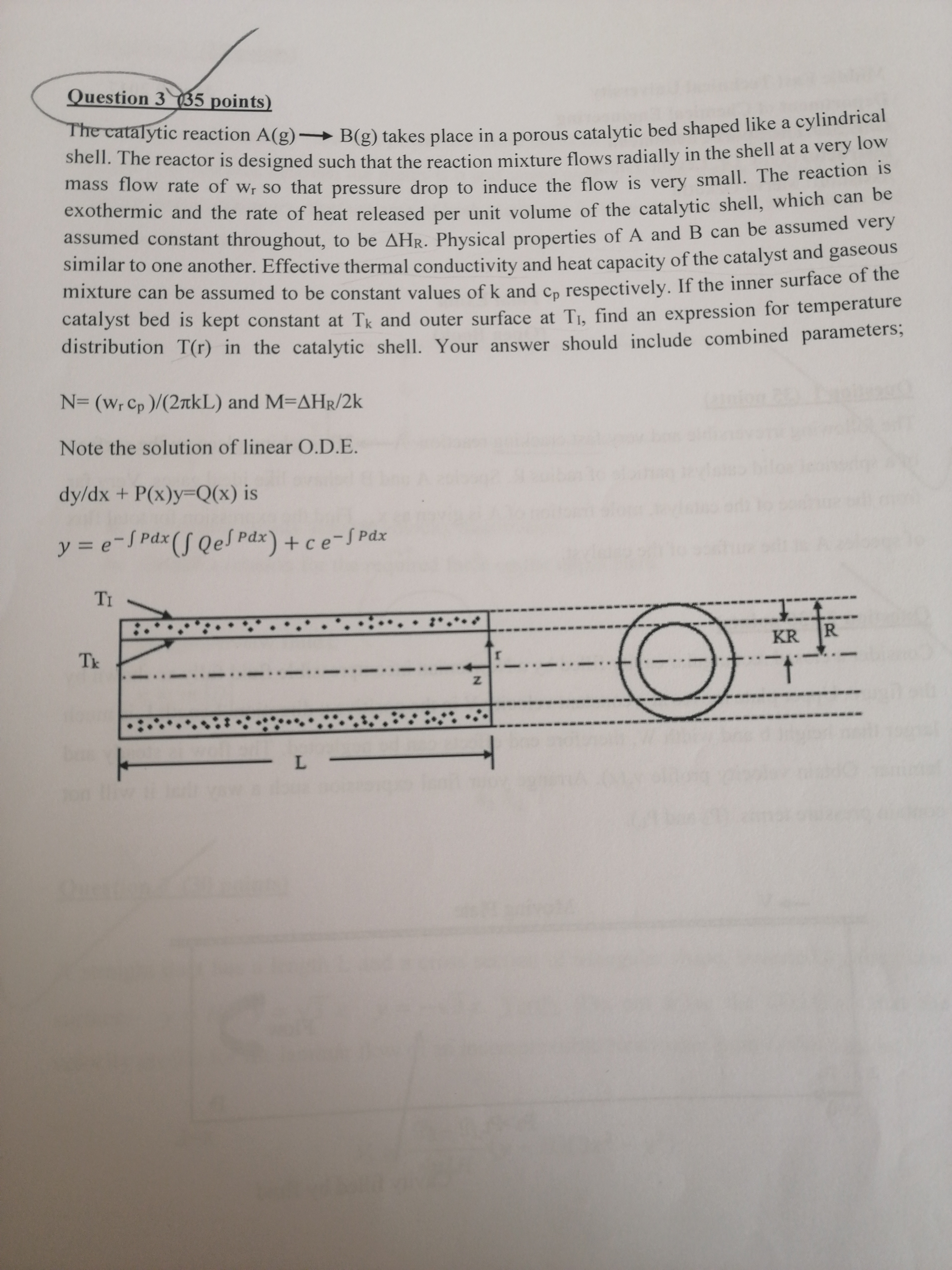
Question: Question 3 ( 3 5 points ) The catalytic reaction A ( g ) l o n g r i g h t a r
Question points
The catalytic reaction takes place in a porous catalytic bed shaped like a cylindrical
shell. The reactor is designed such that the reaction mixture flows radially in the shell at a very low
mass flow rate of so that pressure drop to induce the flow is very small. The reaction is
exothermic and the rate of heat released per unit volume of the catalytic shell, which can be
assumed constant throughout, to be Physical properties of A and can be assumed very
similar to one another. Effective thermal conductivity and heat capacity of the catalyst and gaseous
mixture can be assumed to be constant values of and respectively. If the inner surface of the
catalyst bed is kept constant at and outer surface at find an expression for temperature
distribution in the catalytic shell. Your answer should include combined parameters;
and
Note the solution of linear ODE
is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


