Question: question 3, 4, 5, & 6 using the data given. trials 1,2 & 3 were room temp and trial 4 was in hot water bath
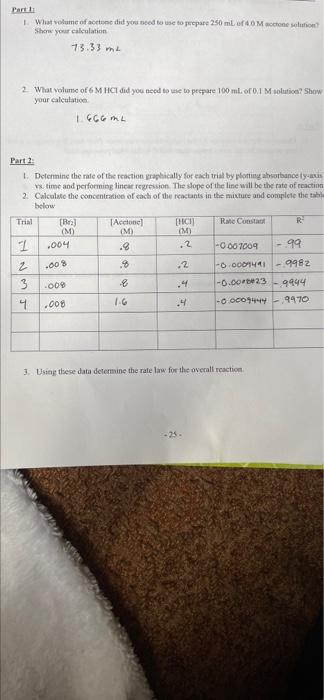

Parti What volume of action did you need to use to prepare 250 ml of Mone solution Show your calculation 73.33 m 2 What volume of 6 M HCI did you need to use to prepare 100 ml. of 0,1 M solutical Show your calculation 1666 Part 2 1. Determine the rate of the reaction graphically for each trial by plotting absorbance lys s time and performing linear regression. The slope of the line will be the rate of reaction 2 Calculate the concentration of each of the reactants in the mixture and complete the table below Trial [B Acetone) [HACIJ Rahe Const R M M (M I ,004 .8 .2 -0.007009 -99 2 % 2 -O DOO -9982 3 009 8 .4 -0.00-23 9444 IG 4 -0.00074449470 .00% .00 3. Using these data determine the ralelow for the overall reaction -25. Chemistry Lab CHEM 112 4 Determine the rate constant for each room temperature experiment is the rate constant truly constant? What do you observe about the rate constant Part 5. Determine the rate constant for the higher temperature trials. How does this compare to the rate constant for the room temperature trial is this what you expected 6. Using the rate constants from the room temperature trials and heated trials determine the activation energy for this reaction Parti What volume of action did you need to use to prepare 250 ml of Mone solution Show your calculation 73.33 m 2 What volume of 6 M HCI did you need to use to prepare 100 ml. of 0,1 M solutical Show your calculation 1666 Part 2 1. Determine the rate of the reaction graphically for each trial by plotting absorbance lys s time and performing linear regression. The slope of the line will be the rate of reaction 2 Calculate the concentration of each of the reactants in the mixture and complete the table below Trial [B Acetone) [HACIJ Rahe Const R M M (M I ,004 .8 .2 -0.007009 -99 2 % 2 -O DOO -9982 3 009 8 .4 -0.00-23 9444 IG 4 -0.00074449470 .00% .00 3. Using these data determine the ralelow for the overall reaction -25. Chemistry Lab CHEM 112 4 Determine the rate constant for each room temperature experiment is the rate constant truly constant? What do you observe about the rate constant Part 5. Determine the rate constant for the higher temperature trials. How does this compare to the rate constant for the room temperature trial is this what you expected 6. Using the rate constants from the room temperature trials and heated trials determine the activation energy for this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


