Question: Question 3 (8 marks) The gas-phase reaction is shown as 3A(g)+B(g)4C(g)+2D(g) Table 2 shows the thermodynamic property of the components involve in the reaction. Assume
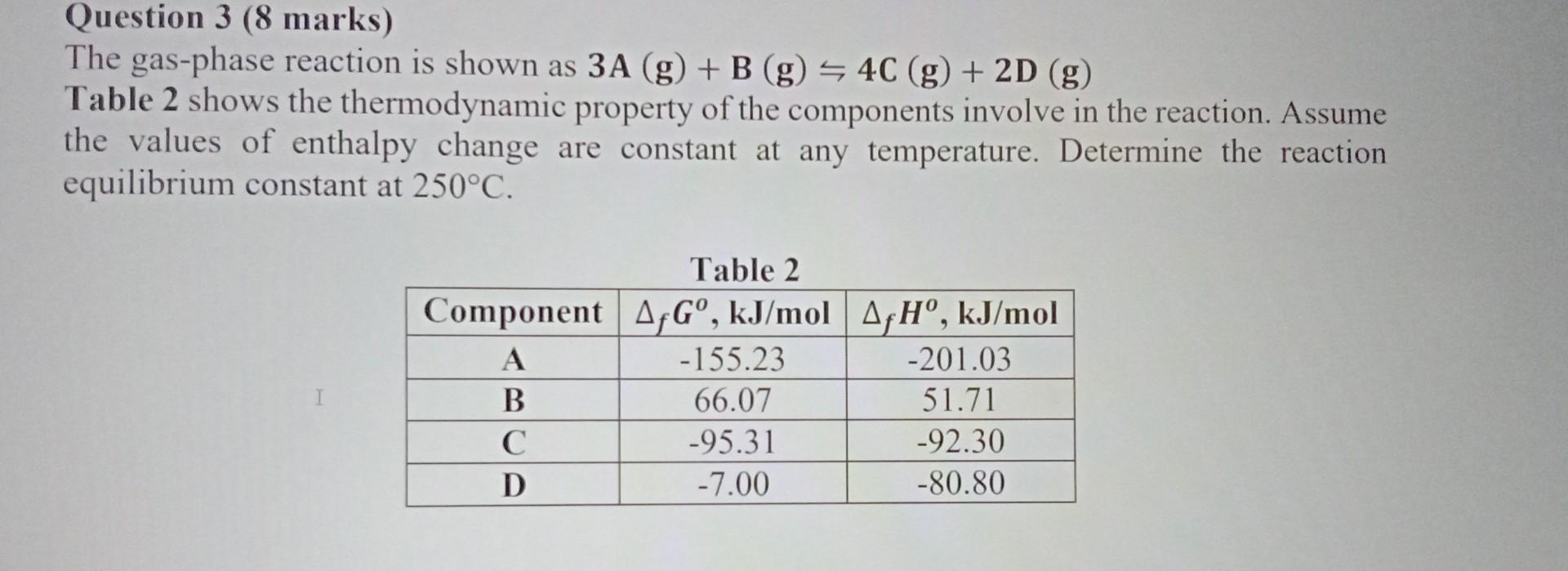
Question 3 (8 marks) The gas-phase reaction is shown as 3A(g)+B(g)4C(g)+2D(g) Table 2 shows the thermodynamic property of the components involve in the reaction. Assume the values of enthalpy change are constant at any temperature. Determine the reaction equilibrium constant at 250C
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


