Question: Question 3) A+BC+D reaction takes place adiabatically in a fully stirred continuous reaclor (CSTR). The feed is equimolar and the concentrations of A and B
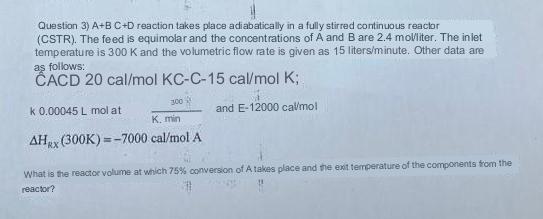
Question 3) A+BC+D reaction takes place adiabatically in a fully stirred continuous reaclor (CSTR). The feed is equimolar and the concentrations of A and B are 2.4 molliter. The iniet temperature is 300K and the volumetric flow rate is given as 15 liters/minute. Other data are as follows: ACD 20cal/molKCC15cal/molK; k0.00045L mol at K,min300+1 and E12000calmol HRX(300K)=7000cal/molA What is the reactor volume at which 75% conversion of A takes place and the exit temperature of the components thom the reactor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


