Question: Question 3 : Ammonium Nitrate ( N H 4 N O 3 ) is a form of fertiliser which can also be used as an
Question :
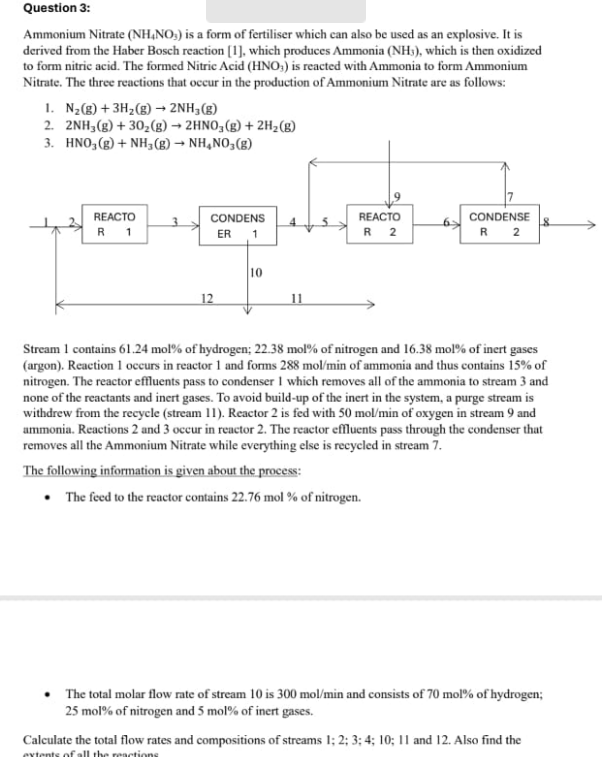
Ammonium Nitrate is a form of fertiliser which can also be used as an explosive. It is
derived from the Haber Bosch reaction which produces Ammonia which is then oxidized
to form nitric acid. The formed Nitric Acid is reacted with Ammonia to form Ammonium
Nitrate. The three reactions that occur in the production of Ammonium Nitrate are as follows:
Stream contains mol of hydrogen; mol of nitrogen and mol of inert gases
argon Reaction occurs in reactor and forms of ammonia and thus contains of
nitrogen. The reactor effluents pass to condenser which removes all of the ammonia to stream and
none of the reactants and inert gases. To avoid buildup of the inert in the system, a purge stream is
withdrew from the recycle stream Reactor is fed with of oxygen in stream and
ammonia. Reactions and occur in reactor The reactor effluents pass through the condenser that
removes all the Ammonium Nitrate while everything else is recycled in stream
The following information is given about the process:
The feed to the reactor contains mol of nitrogen.
The total molar flow rate of stream is and consists of mol of hydrogen;
mol of nitrogen and mol of inert gases.
Calculate the total flow rates and compositions of streams ;;;;; and Also find the
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


