Question: QUESTION # 3. At a point in a wetted-wall column for the absorption of SO2(A) from gas stream using water (B) the bulk concentration is
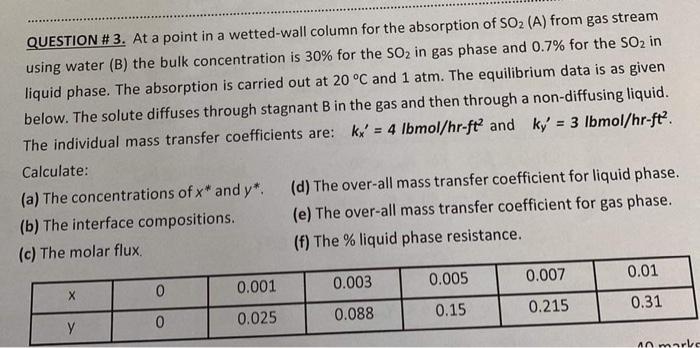
QUESTION \# 3. At a point in a wetted-wall column for the absorption of SO2(A) from gas stream using water (B) the bulk concentration is 30% for the SO2 in gas phase and 0.7% for the SO2 in liquid phase. The absorption is carried out at 20C and 1atm. The equilibrium data is as given below. The solute diffuses through stagnant B in the gas and then through a non-diffusing liquid. The individual mass transfer coefficients are: kx=4lbmol/hrft2 and ky=3lbmol/hrft2. Calculate: (a) The concentrations of x and y. (d) The over-all mass transfer coefficient for liquid phase. (b) The interface compositions. (e) The over-all mass transfer coefficient for gas phase. (c) The molar flux. (f) The \% liquid phase resistance
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


