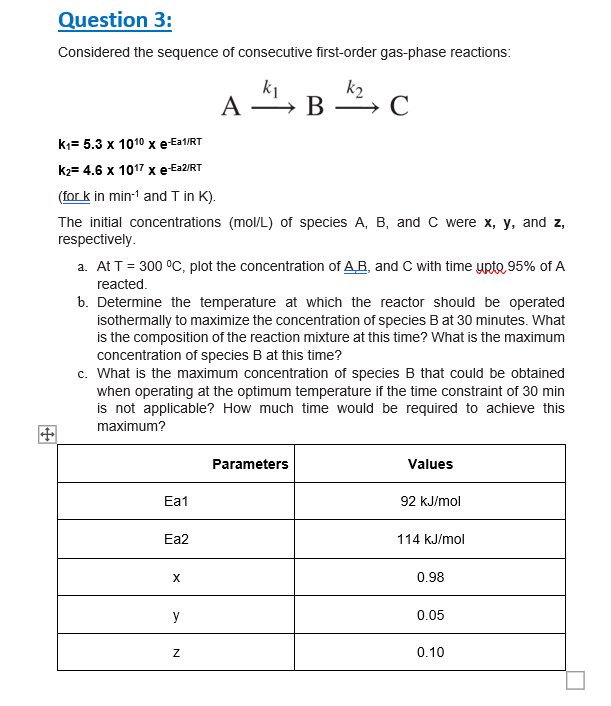
Question: Question 3 : Considered the sequence of consecutive first - order gas - phase reactions: A k 1 B k 2 C k 1 =
Step by Step Solution
There are 3 Steps involved in it
To solve this problem well follow these steps Step 1 Determine Rate Constants at 300C 1 Convert 300C ... View full answer

Get step-by-step solutions from verified subject matter experts


