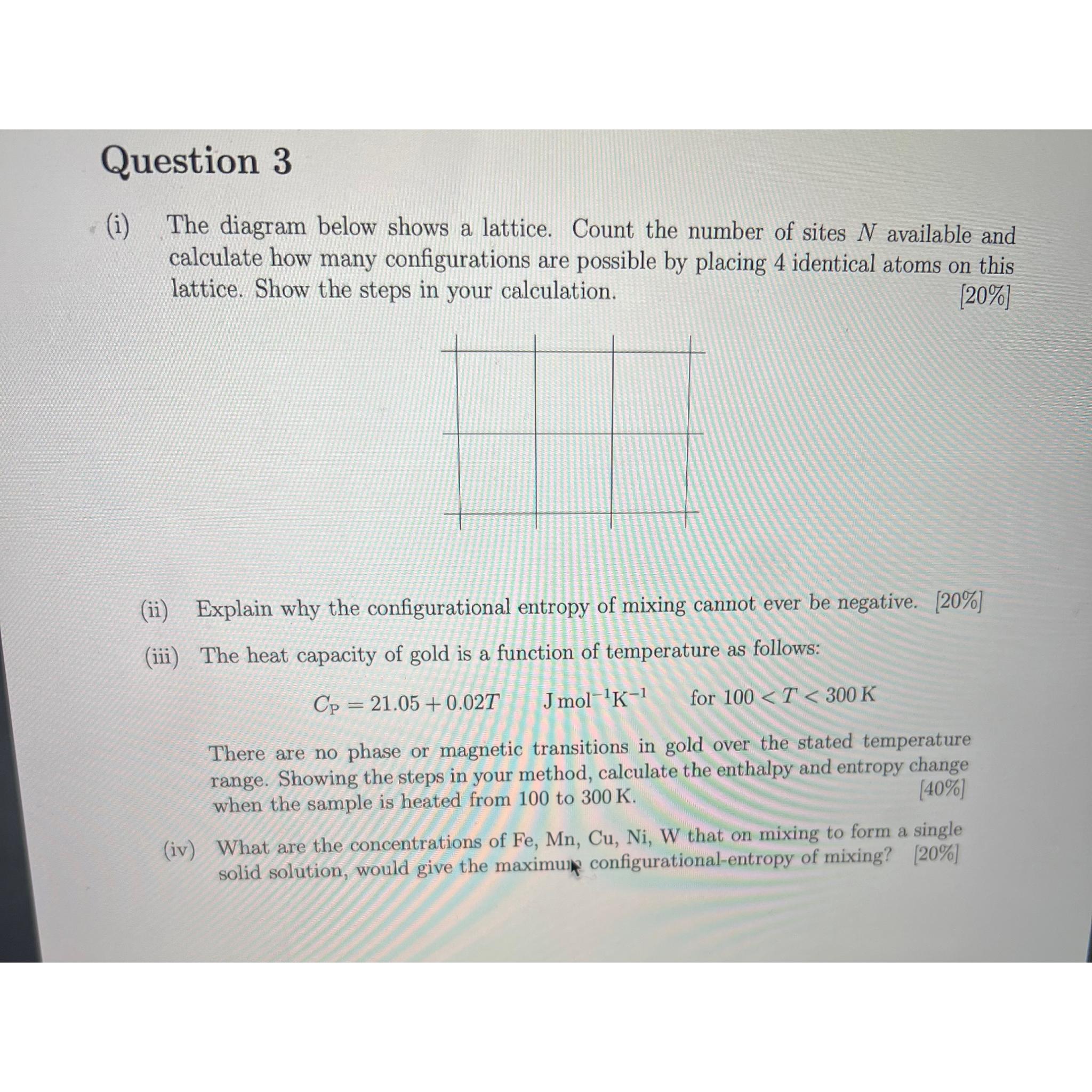
Question: Question 3 ( i ) The diagram below shows a lattice. Count the number of sites N available and calculate how many configurations are possible
Question
i The diagram below shows a lattice. Count the number of sites available and calculate how many configurations are possible by placing identical atoms on this lattice. Show the steps in your calculation.
ii Explain why the configurational entropy of mixing cannot ever be negative.
iii The heat capacity of gold is a function of temperature as follows:
KFe, for
There are phase magnetic transitions gold over the stated temperature range. Showing the steps your method, calculate the enthalpy and entropy change when the sample heated from
What are the concentrations that mixing form a single solid solution, would give the maximul configurationalentropy mixing?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


