Question: Question 3 Phase diagrams (4 marks) Using the following phase diagram of the Magnesium (Mg) -Lead (Pb) in the Figure 3 Composition (at %
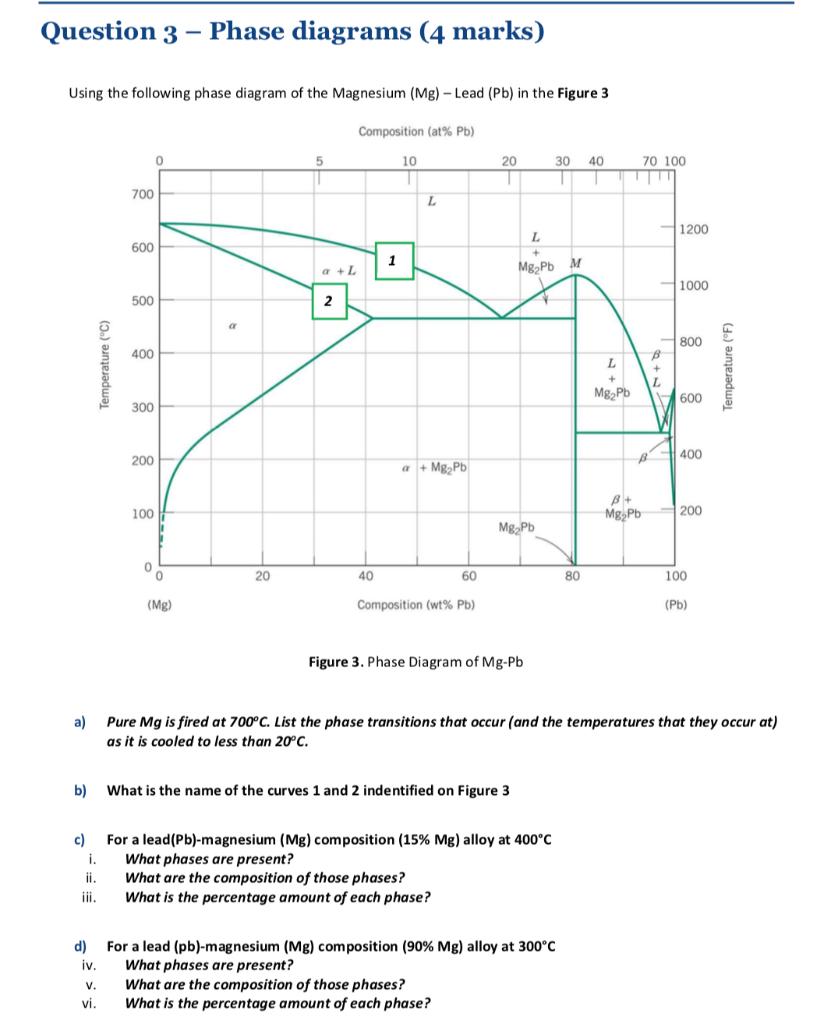
Question 3 Phase diagrams (4 marks) Using the following phase diagram of the Magnesium (Mg) -Lead (Pb) in the Figure 3 Composition (at % Pb) a) c) i. iii. d) iv. Temperature (C) V. vi. 700 600 500 400 300 200 100 0 0 0 (Mg) a 20 a + L 2 40 1 10 L a+MgPb b) What is the name of the curves 1 and 2 indentified on Figure 3 60 Composition (wt% Pb) 20 MgPb Figure 3. Phase Diagram of Mg-Pb L + MgPb M What are the composition of those phases? What is the percentage amount of each phase? 30 40 T T For a lead (Pb)-magnesium (Mg) composition ( 15% Mg) alloy at 400C What phases are present? What are the composition of those phases? What is the percentage amount of each phase? For a lead (pb)-magnesium (Mg) composition (90% Mg) alloy at 300C What phases are present? 80 L + MgPb 70 100. B + L 1 B 1200 1000 800 600 400 Pure Mg is fired at 700C. List the phase transitions that occur (and the temperatures that they occur at) as it is cooled to less than 20C. B+ MgPb 200 100 (Pb) Temperature (F)
Step by Step Solution
3.36 Rating (149 Votes )
There are 3 Steps involved in it
3 Lad liquidus line solidus line 2 154 75 at 40... View full answer

Get step-by-step solutions from verified subject matter experts


