Question: Question 3: The following three mechanical reversible processes are applied to air assumed to be an ideal gas: heating at constant volume (Process 1-2), expansion
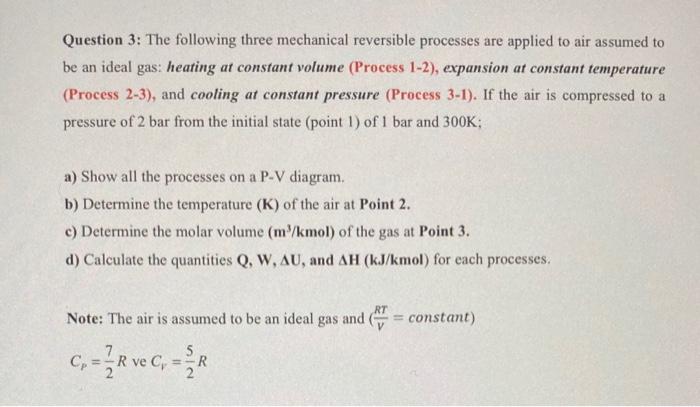
Question 3: The following three mechanical reversible processes are applied to air assumed to be an ideal gas: heating at constant volume (Process 1-2), expansion at constant temperature (Process 2-3), and cooling at constant pressure (Process 3-1). If the air is compressed to a pressure of 2 bar from the initial state (point 1 ) of 1 bar and 300K; a) Show all the processes on a P-V diagram. b) Determine the temperature (K) of the air at Point 2. c) Determine the molar volume (m3/kmol) of the gas at Point 3 . d) Calculate the quantities Q,W,U, and H(kJ/kmol) for each processes. Note: The air is assumed to be an ideal gas and (VRT= constant ) CP=27R ve CV=25R Question 3: The following three mechanical reversible processes are applied to air assumed to be an ideal gas: heating at constant volume (Process 1-2), expansion at constant temperature (Process 2-3), and cooling at constant pressure (Process 3-1). If the air is compressed to a pressure of 2 bar from the initial state (point 1 ) of 1 bar and 300K; a) Show all the processes on a P-V diagram. b) Determine the temperature (K) of the air at Point 2. c) Determine the molar volume (m3/kmol) of the gas at Point 3 . d) Calculate the quantities Q,W,U, and H(kJ/kmol) for each processes. Note: The air is assumed to be an ideal gas and (VRT= constant ) CP=27R ve CV=25R
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


