Question: Question 3 (to point) Which combination would you predict to not be soluble in exch ceche a. CH2NOH2 and H2O bi CH3CH2CH3 and CH3CH2CH2F G.
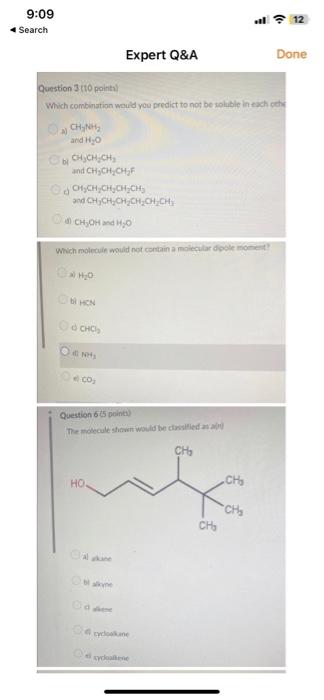

Question 3 (to point) Which combination would you predict to not be soluble in exch ceche a. CH2NOH2 and H2O bi CH3CH2CH3 and CH3CH2CH2F G. CH2CH2CH2CH2CH3 and CH3CH2CH2CH2CH2CH1 b) CH2OH and H2O Which molecuin would not contain a moitenlar dople monent? (2) H2O bi hen CCHCl2 (fi Na4 cos2 Question 655 penbi) The molecule stownt woild be dissilied at aliv at ane bi atine at ares tis swiopatene 2uestion 7 (10 points) Which of the following would you predict to be the most soluble in water? a) CH3COCH3 b) CH3CH2OCH3 c) CH3CH2COOH d) CH2CH2CHO Question 8(5 points) Which of the following would be the strongest acid? a) H2O b) H2S c) HBr d) HCl Question 9 tio pointd Which of the following wouls vou presict to huve the higher boilins point? bi)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


