Question: Pure caffeine has a melting point of around 235 C. Suppose after you isolated your caffeine from coffee, you found that your sample had
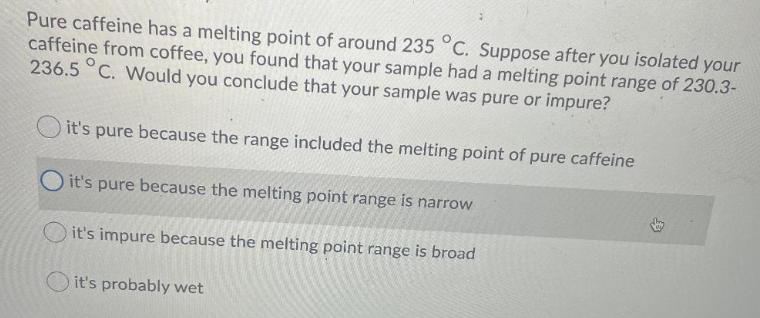
Pure caffeine has a melting point of around 235 C. Suppose after you isolated your caffeine from coffee, you found that your sample had a melting point range of 230.3- 236.5 C. Would you conclude that your sample was pure or impure? it's pure because the range included the melting point of pure caffeine Oit's pure because the melting point range is narrow it's impure because the melting point range is broad it's probably wet
Step by Step Solution
There are 3 Steps involved in it
Question 391 has a sharp melt A Compound material shapp melting point if the mange of m... View full answer

Get step-by-step solutions from verified subject matter experts


