Question: Question 4 1 pts Below is the fuel mixture consisting of 0.2 moles of heptane (C7H16), 0.6 moles of hexane (C6H14), and 1.2 moles of
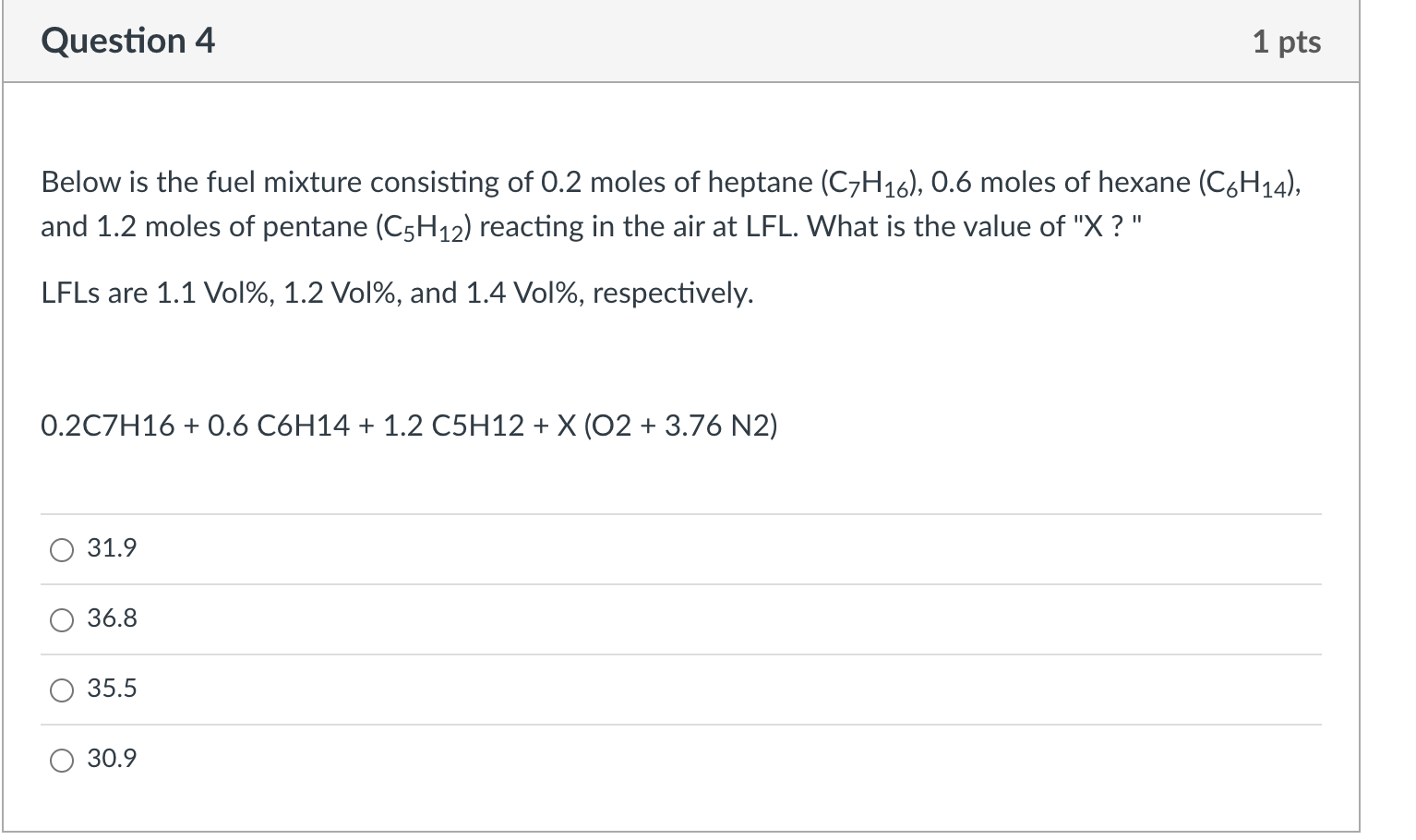
Question 4 1 pts Below is the fuel mixture consisting of 0.2 moles of heptane (C7H16), 0.6 moles of hexane (C6H14), and 1.2 moles of pentane (C5H12) reacting in the air at LFL. What is the value of "X ?" LFLs are 1.1 Vol%, 1.2 Vol%, and 1.4 Vol%, respectively. 0.2C7H16 + 0.6 C6H14 + 1.2 C5H12 + X (O2 + 3.76 N2) 31.9 36.8 35.5 30.9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


