Question: Question 4 ( 2 0 marks ) ( a ) Nitrogen ( left ( mathrm { N } _ { 2 }
Question marks
a Nitrogen leftmathrmNright in the air may dissociate to very reactive monatomic nitrogen N under high temperatures, leading to formation of NOx. Please calculate the percentage of mathrmN that dissociates to N in the reaction mathbfNmathbfrightarrow mathbf N at temperature and pressure of K and kPa
marks
b Propose one method to reduce the formation of mathrmNOmathrmx in IC engines with justification and explain its effect on engine performance.
marks
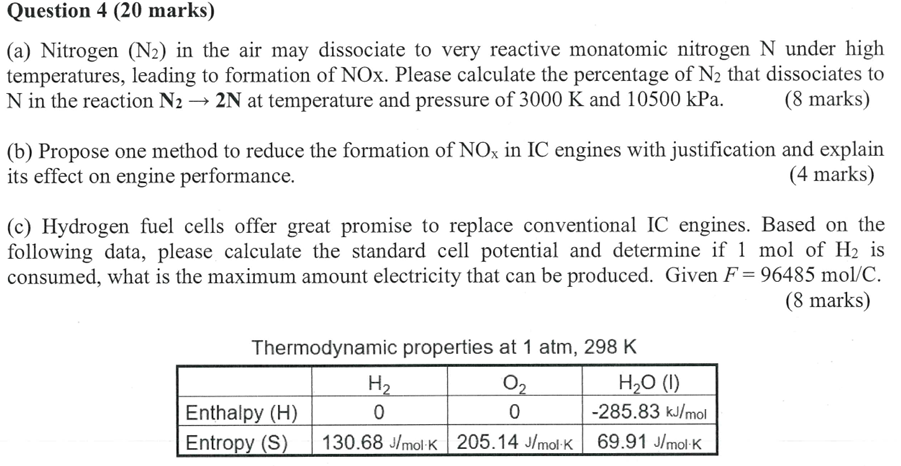
c Hydrogen fuel cells offer great promise to replace conventional IC engines. Based on the following data, please calculate the standard cell potential and determine if mol of mathrmH is consumed, what is the maximum amount electricity that can be produced. Given Fmathrm~molmathrmC
marks
Thermodynamic properties at mathrm~atmmathrm~K
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


