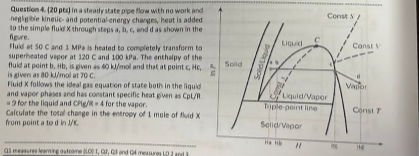
Question: Question 4 . ( 2 0 pts ) in a steady state pipe flow with no work and negligible kinetic - and potential energy charges,
Question pts in a steady state pipe flow with no work and
negligible kineticand potential energy charges, heat is added
to the simple fluid through steps b c end d as shown in the
figure. fluld at and MPa is heated to completely transform to
superheated vapor at and kPa. The enthalpy of the
fluld at point b Hb is geven as and that at point c Hc
is given as kjmoll at
Fiuid follows the ideal gas equation of state both in the liquld
and wapor phesas and has constant specific heat given as CpLR
for the liquid and CpVR for the vapor.
Caiculate the total change in the entropy of mole of fluid
from point a to d in J
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


