Question: For a binary system consists of the cyclopentane (species 1) and cyclohexane (species 2), this binary system is found to obey Raoult's law. It
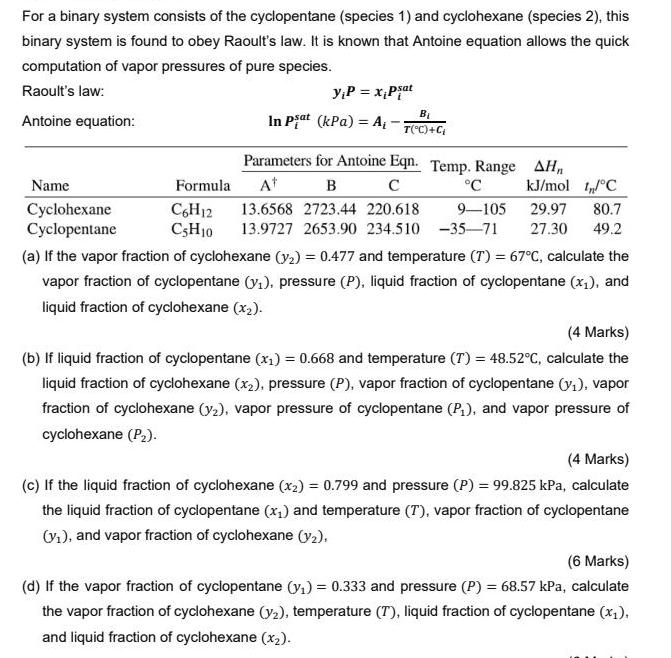
For a binary system consists of the cyclopentane (species 1) and cyclohexane (species 2), this binary system is found to obey Raoult's law. It is known that Antoine equation allows the quick computation of vapor pressures of pure species. Raoult's law: yP = x,Pjat Antoine equation: In Piat (kPa) = A, - T("C)+C Parameters for Antoine Eqn. Temp. Range AHn Name Formula At C kJ/mol IC Cyclohexane Cyclopentane C,H12 CSH10 13.6568 2723.44 220.618 9-105 29.97 80.7 13.9727 2653.90 234.510 -35-71 27.30 49.2 (a) If the vapor fraction of cyclohexane (y2) = 0.477 and temperature (T) = 67C, calculate the vapor fraction of cyclopentane (y,), pressure (P), liquid fraction of cyclopentane (x1), and liquid fraction of cyclohexane (x2). (4 Marks) (b) If liquid fraction of cyclopentane (x,) = 0.668 and temperature (T) = 48.52c, calculate the liquid fraction of cyclohexane (x2), pressure (P), vapor fraction of cyclopentane (y1), vapor fraction of cyclohexane (y2), vapor pressure of cyclopentane (P,), and vapor pressure of cyclohexane (P2). (4 Marks) (c) If the liquid fraction of cyclohexane (x2) = 0.799 and pressure (P) = 99.825 kPa, calculate the liquid fraction of cyclopentane (x,) and temperature (T), vapor fraction of cyclopentane Vi), and vapor fraction of cyclohexane (y2). (6 Marks) (d) If the vapor fraction of cyclopentane (y,) = 0.333 and pressure (P) = 68.57 kPa, calculate the vapor fraction of cyclohexane (y2), temperature (T), liquid fraction of cyclopentane (x;), and liquid fraction of cyclohexane (x2).
Step by Step Solution
3.18 Rating (146 Votes )
There are 3 Steps involved in it
solution In part c and part d temperature... View full answer

Get step-by-step solutions from verified subject matter experts


