Question: Question 4 3 Incorrect Mark 0 . 0 0 out of 1 . 0 0 Question 4 4 Not answered Marked out of 1 .
Question
Incorrect
Mark out of
Question
Not answered
Marked out of
For the decomposition of reactant determine the order of the reaction from the concentrationtime dependence given in the next question.
Answer:
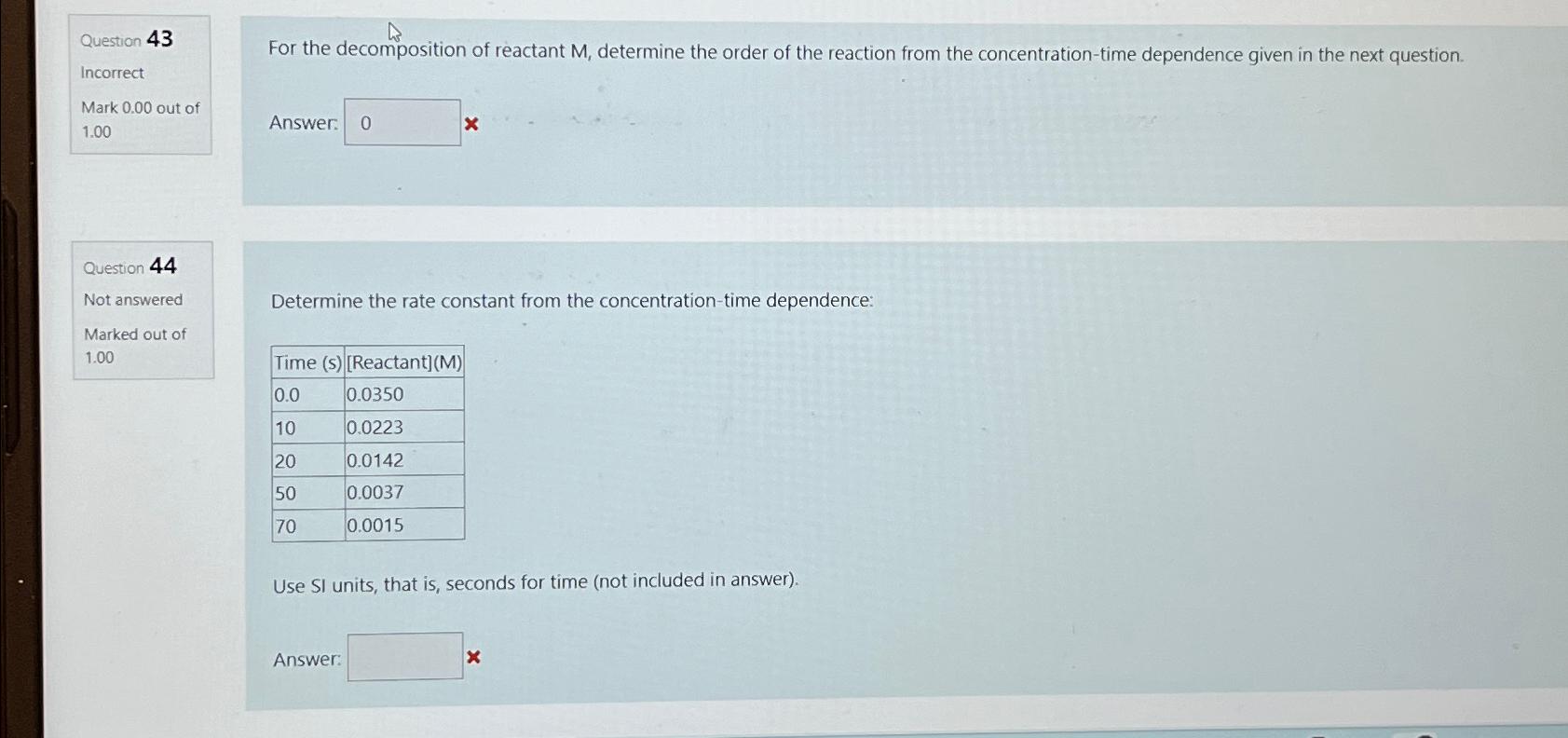
Determine the rate constant from the concentrationtime dependence:
tableTime sReactantM
Use SI units, that is seconds for time not included in answer
Answer:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


