Question: question 4 ( 6 points ) For complete stoichiometric combustion of gasoline, C 7 H 1 7 , determine the fuel molecular weight, the combustion
question points
For complete stoichiometric combustion of gasoline, determine the fuel molecular weight, the combustion products, and the mass of carbon dioxide produced per kilogram of fuel burned. The stoichiometric combustion gives:
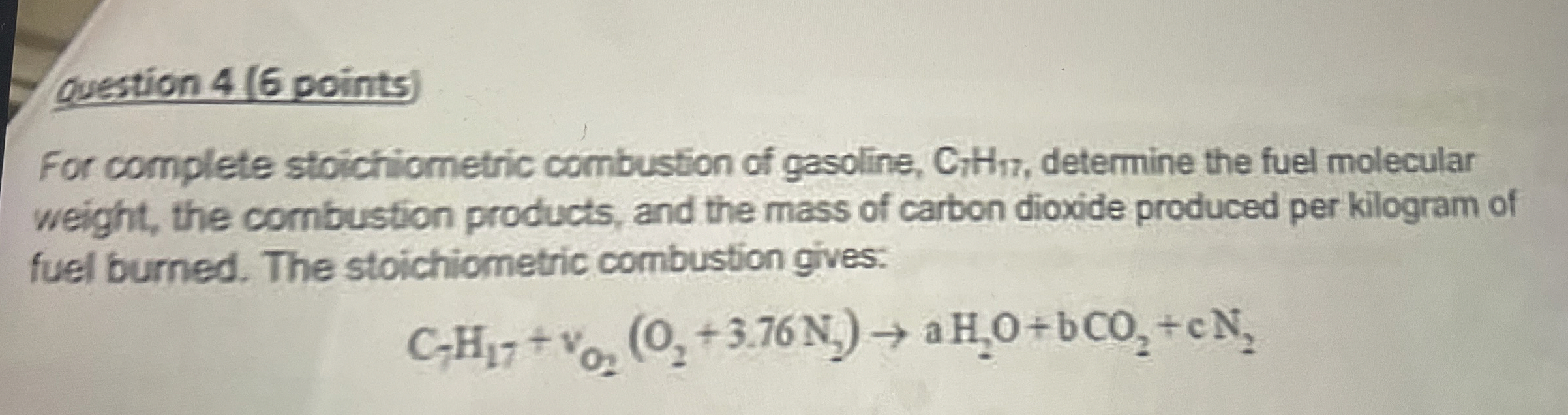
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


