Question: Question 4 . ( a ) Discuss how chemical and enzymatic kinetics are relevant to transport phenomena. ( b ) What are the dimensions of
Question
a Discuss how chemical and enzymatic kinetics are relevant to transport phenomena.
b What are the dimensions of the reaction rate constants for zeroth first and second
order reactions? Are they the same?
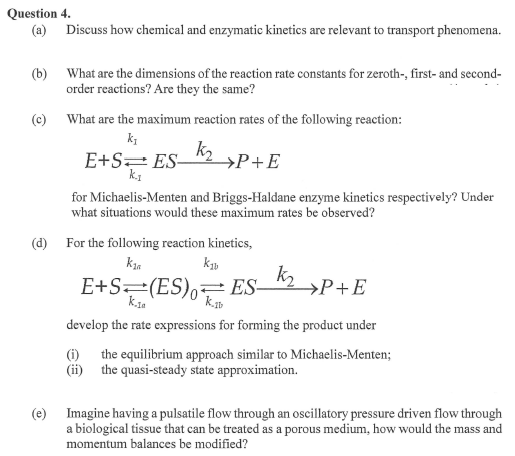
c What are the maximum reaction rates of the following reaction:
for MichaelisMenten and BriggsHaldane enzyme kinetics respectively? Under
what situations would these maximum rates be observed?
d For the following reaction kinetics,
develop the rate expressions for forming the product under
i the equilibrium approach similar to MichaelisMenten;
ii the quasisteady state approximation.
e Imagine having a pulsatile flow through an oscillatory pressure driven flow through
a biological tissue that can be treated as a porous medium, how would the mass and
momentum balances be modified?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


