Question: QUESTION 4 : Combustion ( 2 5 Marks ) A sample of biogas from a renewable energy project is 8 0 % methane ( C
QUESTION : Combustion Marks
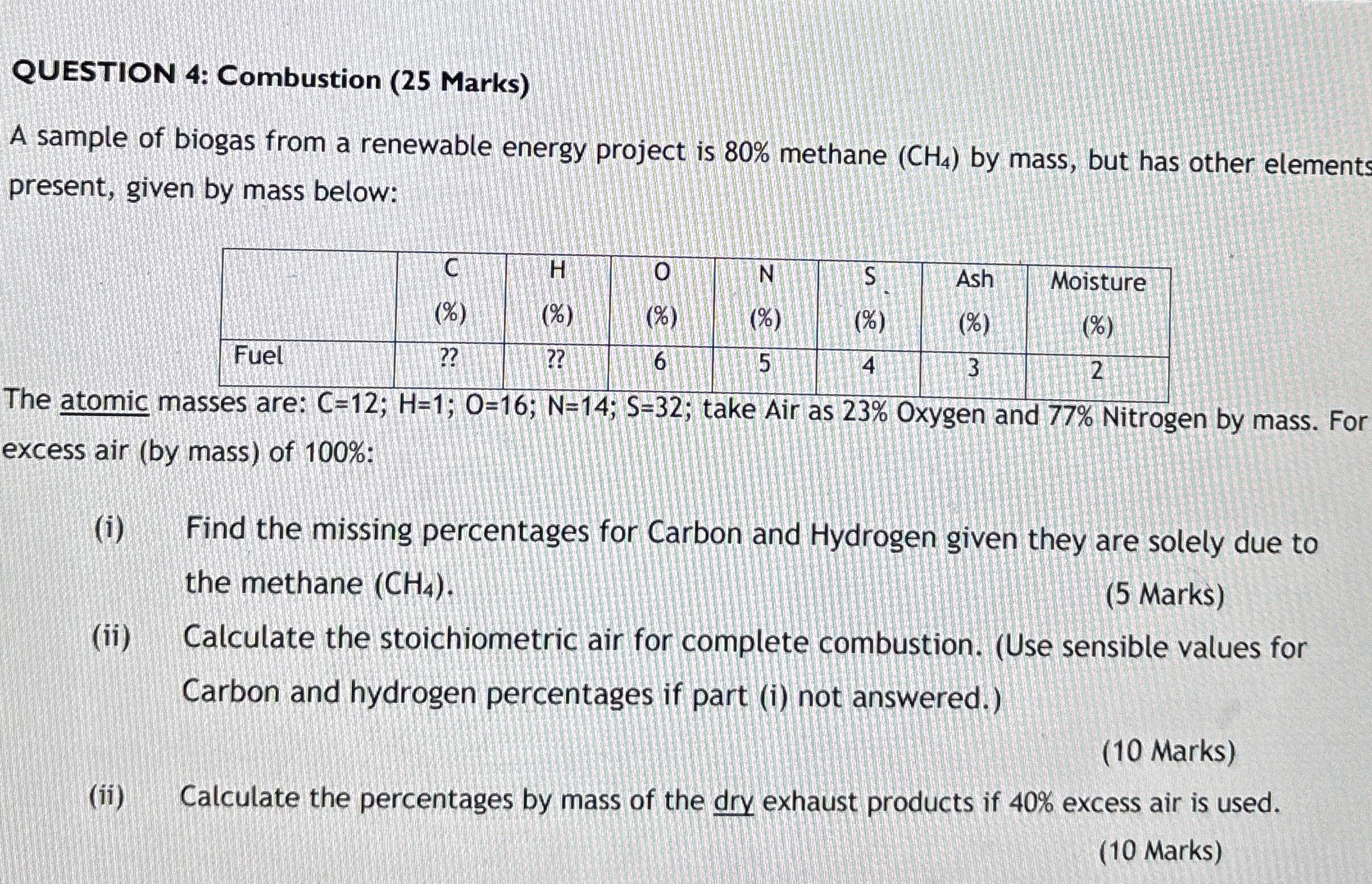
A sample of biogas from a renewable energy project is methane by mass, but has other element present, given by mass below:
tableCHONSAsh,MoistureFuel
The atomic masses are: ;;;;; take Air as Oxygen and Nitrogen by mass. For excess air by mass of :
i Find the missing percentages for Carbon and Hydrogen given they are solely due to the methane
Marks
ii Calculate the stoichiometric air for complete combustion. Use sensible values for Carbon and hydrogen percentages if part i not answered.
Marks
ii Calculate the percentages by mass of the dry exhaust products if excess air is used.
Marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


