Question: QUESTION 4 |Marks 201 A reversible gas phase reaction: 24 B+C is to be carried out at 1033 K and pressure of 506.6 kPa. The
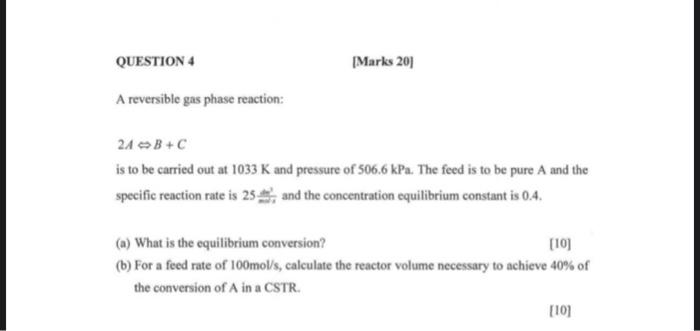
QUESTION 4 |Marks 201 A reversible gas phase reaction: 24 B+C is to be carried out at 1033 K and pressure of 506.6 kPa. The feed is to be pure A and the specific reaction rate is 25, and the concentration equilibrium constant is 0.4. (a) What is the equilibrium conversion? [10] (b) For a feed rate of 100mol/s, calculate the reactor volume necessary to achieve 40% of the conversion of A in a CSTR. [101
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


