Question: question 4 please (product A can be seen below) and it is SN2 2. Based on your A:B product ratio which mechanism, Sn1or Sn2, is
question 4 please (product A can be seen below) and it is SN2
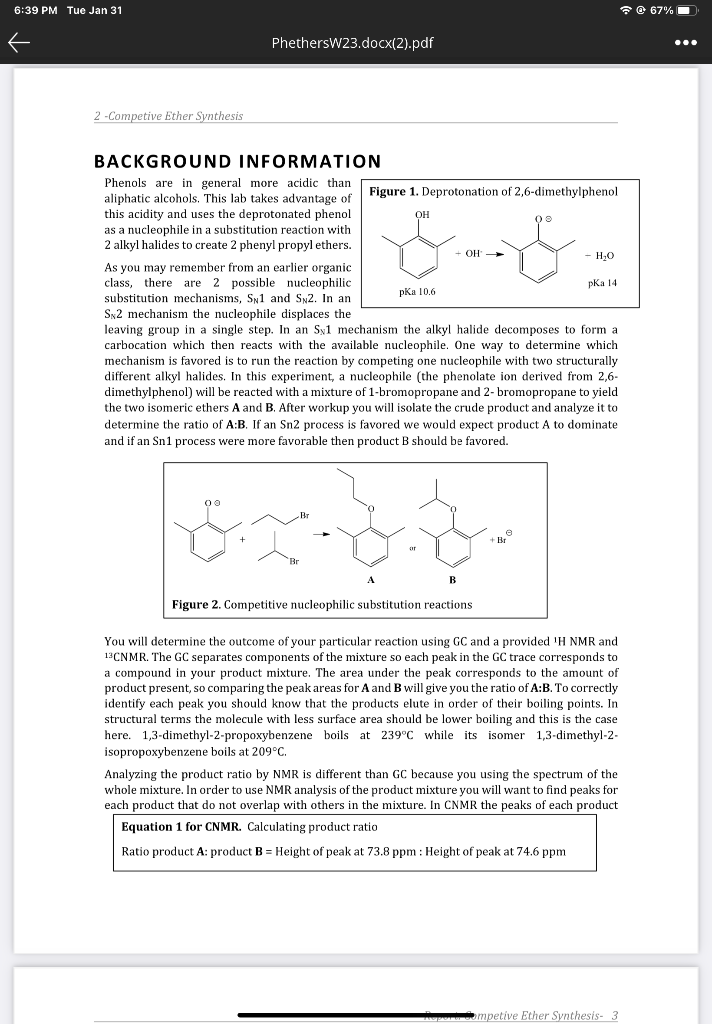
2. Based on your A:B product ratio which mechanism, Sn1or Sn2, is implicated? Explain 2pts Sn 2 because prodwet A is more stable and has lower imfs, nueleophiledisplaces leaving groupin single step. Report: Competive Ether Synthesis- 5 3. Consider the solvent, ethanol, used in this reaction. Is this solvent protic or aprotic? Is the solvent choice consistent with the mechanism you concluded above? 2pts Ethanolis a polar protic solwent, it is not consistent with the sne mechanism becawse it car hyorogen bond to nutleophiles and maitethem iess effective in attatuing the subjtrate. 4. Using the mechanism implicated in Q\#2 above draw the stepwise mechanism for the reaction to produce the product A. Begin your mechanism with the 2,6-dimethylphenol reacting with sodium hydroxide to form the 2,6-dimethylphenolate ion then show the mechanism for ether formation. show electron movement with arrows in each step. 3pts 2ib-dimethyl phenor 2, 6-dimethyl phenolate ion BACKGROUND INFORMATION Phenols are in general more acidic than aliphatic alcohols. This lab takes advantage of this acidity and uses the deprotonated phenol as a nucleophile in a substitution reaction with 2 alkyl halides to create 2 phenyl propyl ethers. As you may remember from an earlier organic class, there are 2 possible nucleophilic substitution mechanisms, SN1 and SN2. In an SN2 mechanism the nucleophile displaces the leaving group in a single step. In an SN1 mechanism the alkyl halide decomposes to form a carbocation which then reacts with the available nucleophile. One way to determine which mechanism is favored is to run the reaction by competing one nucleophile with two structurally different alkyl halides. In this experiment, a nucleophile (the phenolate ion derived from 2,6dimethylphenol) will be reacted with a mixture of 1-bromopropane and 2-bromopropane to yield the two isomeric ethers A and B. After workup you will isolate the crude product and analyze it to determine the ratio of A:B. If an Sn2 process is favored we would expect product A to dominate and if an Sn1 process were more favorable then product B should be favored. You will determine the outcome of your particular reaction using GC and a provided 1HNMR and 13CNMR. The GC separates components of the mixture so each peak in the GC trace corresponds to a compound in your product mixture. The area under the peak corresponds to the amount of product present, so comparing the peak areas for A and B will give you the ratio of AB. To correctly identify each peak you should know that the products elute in order of their boiling points. In structural terms the molecule with less surface area should be lower boiling and this is the case here. 1,3-dimethyl-2-propoxybenzene boils at 239C while its isomer 1,3-dimethyl-2isopropoxybenzene boils at 209C. Analyzing the product ratio by NMR is different than GC because you using the spectrum of the whole mixture. In order to use NMR analysis of the product mixture you will want to find peaks for each product that do not overlap with others in the mixture. In CNMR the peaks of each product Equation 1 for CNMR. Calculating product ratio Ratio product A: product B= Height of peak at 73.8ppm : Height of peak at 74.6ppm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


