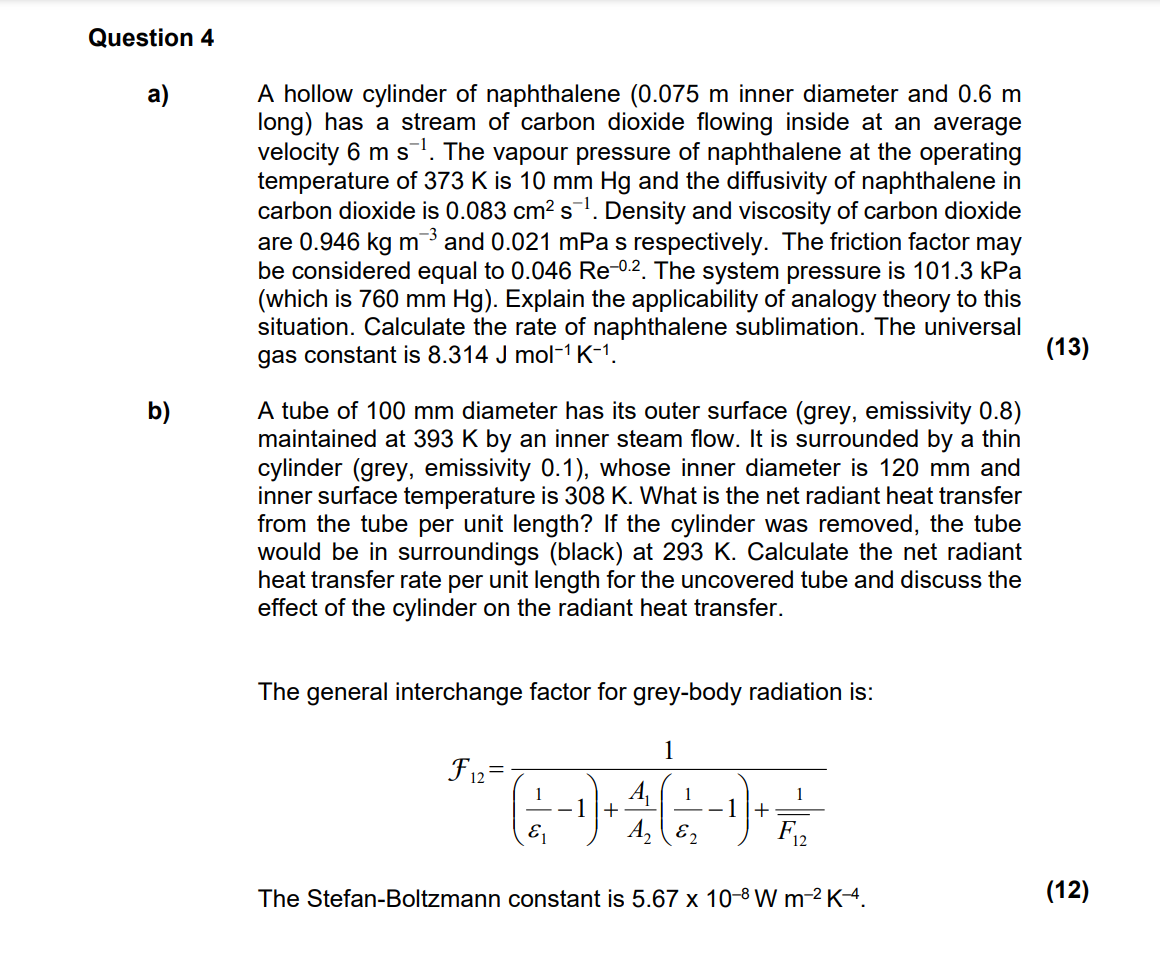
Question: Question 4 ( Please show every step in the process of calcualtions with explanations and assumptions ) a ) A hollow cylinder of naphthalene inner
Question
Please show every step in the process of calcualtions with explanations and assumptions
a A hollow cylinder of naphthalene inner diameter and
long has a stream of carbon dioxide flowing inside at an average
velocity The vapour pressure of naphthalene at the operating
temperature of is and the diffusivity of naphthalene in
carbon dioxide is Density and viscosity of carbon dioxide
are and mPa s respectively. The friction factor may
be considered equal to The system pressure is kPa
which is Explain the applicability of analogy theory to this
situation. Calculate the rate of naphthalene sublimation. The universal
gas constant is
b A tube of diameter has its outer surface grey emissivity
maintained at by an inner steam flow. It is surrounded by a thin
cylinder grey emissivity whose inner diameter is and
inner surface temperature is What is the net radiant heat transfer
from the tube per unit length? If the cylinder was removed, the tube
would be in surroundings black at Calculate the net radiant
heat transfer rate per unit length for the uncovered tube and discuss the
effect of the cylinder on the radiant heat transfer.
The general interchange factor for greybody radiation is:
The StefanBoltzmann constant is
There is a bar on top of the F in the equation. Ps check the imagine for the correct formula.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


