Question: Question 4 Polylactide or polylactic acid (PA) has been able to replace the petroleum based thermoplastics in a large number of applications such as packaging
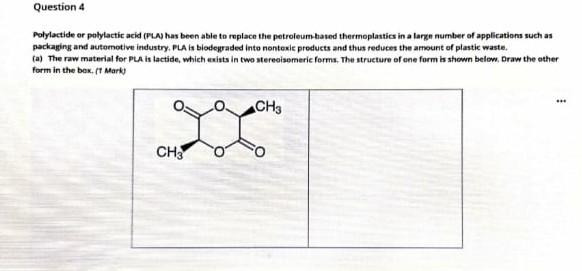

Question 4 Polylactide or polylactic acid (PA) has been able to replace the petroleum based thermoplastics in a large number of applications such as packaging and automotive industry. PLA is blodegraded into nontoxle products and thus reduces the amount of plastic waste. (a) The raw material for PA is lactide, which exists in two stereoisomeric forma. The structure of one form is shown below. Draw the other form in the box. ( Mark 00 CH3 CH3 b) What type of polymerization takes place to produce PLAY (Polymerisation of each steroisomeric lactide produce..... PLA on the other hand, polymerization of racemic mixture of Lactide will give PLA 22 Mark) Lattice can also be copolymerised with Wycolidn to produce palylactide-colycolide PGA Draw a section of the enpolymer showing one repenting unit. (0.5 Mark) . Suggest ane mechanical property in which FLAG differ from PLLA. (0.5 Merk) ii. The bindegradation rate of the homopolymer PLLA and the copolymers PLIGA (PELAJPGA) were found to increase in the following arderPLLAPLAPAS PLLAFGA.PLLAPGAn Explain. Mark te) Nylon 66 and polyte-caprolactone have similar molecular weight and crystallinity. However, the glass transition temperature til for nylon 4,6852 - whereas the Te for pelvie caprolactone) in Draw the structure of the two polymers. Explain the difference in get these two polymers in terms of the intermolecular forces between the chains. 1 Merk
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


