Question: Question 4(Multiple Choice Worth 2 points) (01 07 MC) The pH level, P, of a liquid can be determined from the hydronium ion (HJO*) concentration,
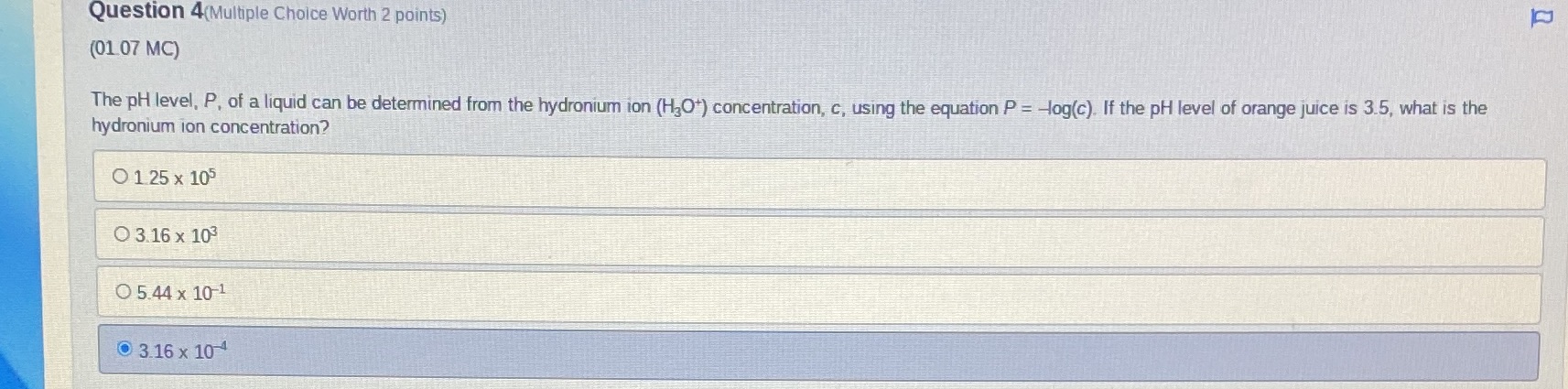
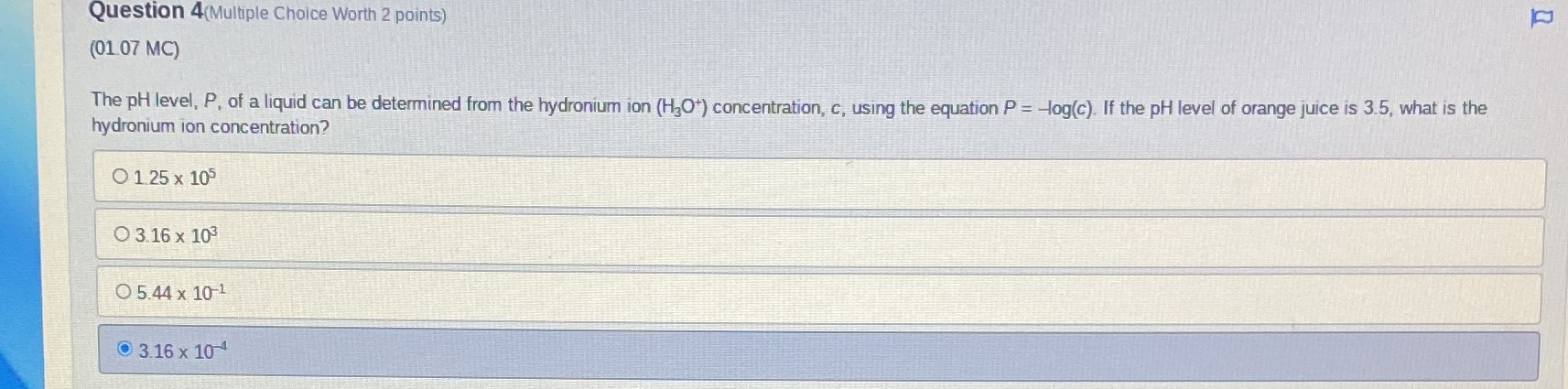
Question 4(Multiple Choice Worth 2 points) (01 07 MC) The pH level, P, of a liquid can be determined from the hydronium ion (HJO*) concentration, c, using the equation P = -log(c). If the pH level of orange juice is 3.5, what is the hydronium ion concentration? O 1 25 x 105 O 3.16 x 103 O 5.44 x 10-1 0 3.16 x 10 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


