Question: Question 5 (1 point) COnsider an ideal gas with initial volume of 5.82E3cm3/mol, pressure P=0.5MPa and Cp=29J/molK. Calculate the entropy change when it expands isothermally
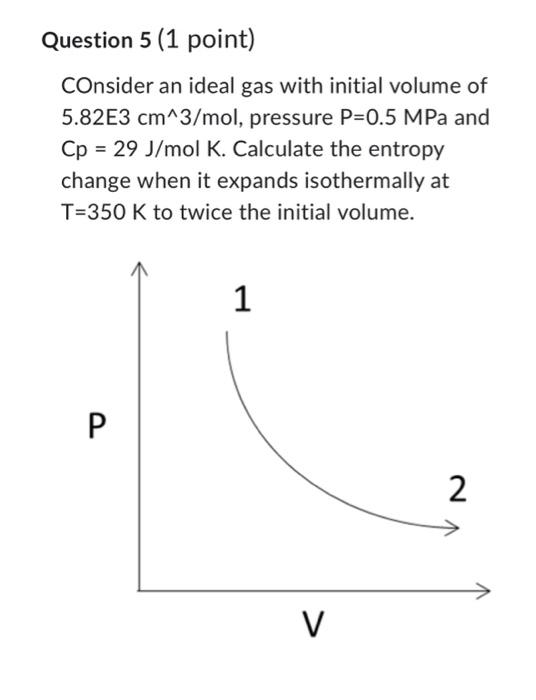
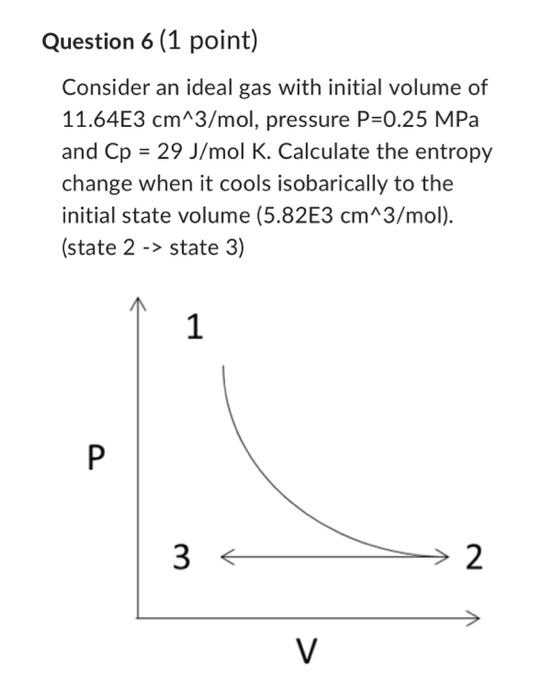
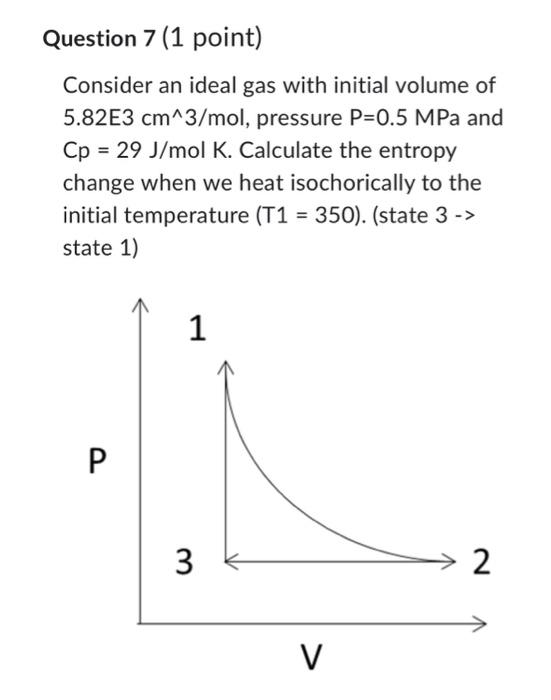
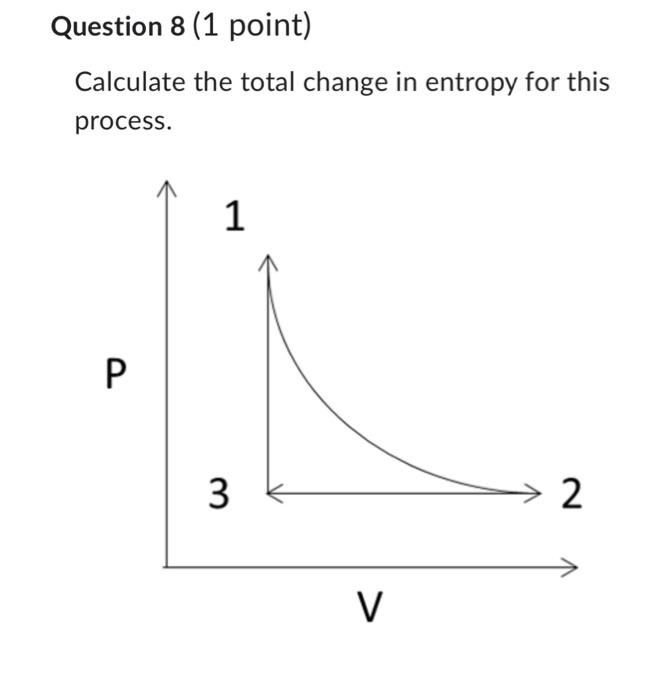
Question 5 (1 point) COnsider an ideal gas with initial volume of 5.82E3cm3/mol, pressure P=0.5MPa and Cp=29J/molK. Calculate the entropy change when it expands isothermally at T=350K to twice the initial volume. Consider an ideal gas with initial volume of 11.64E3 cm3/mol, pressure P=0.25MPa and Cp=29J/molK. Calculate the entropy change when it cools isobarically to the initial state volume (5.82E3 cm3/mol). (state 2 -> state 3 ) Consider an ideal gas with initial volume of 5.82E 3cm3/mol, pressure P=0.5MPa and Cp=29J/molK. Calculate the entropy change when we heat isochorically to the initial temperature (T1=350). (state 3 -> state 1) Question 8 (1 point) Calculate the total change in entropy for this process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


