Question: Question 5: (2 points) The reaction 2NO+O2 + 2NO2 proceeds through the following steps: ki k2 2NO N2O2 (fast) k3 N202+02 2NO2 (slow) Using the
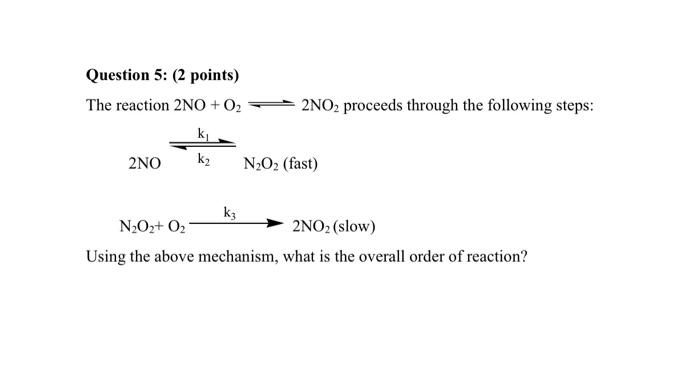
Question 5: (2 points) The reaction 2NO+O2 + 2NO2 proceeds through the following steps: ki k2 2NO N2O2 (fast) k3 N202+02 2NO2 (slow) Using the above mechanism, what is the overall order of reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


