Question: Question 5 2 pts At a particular temperature, Kc = 2.5 x 10-3 for the reaction below: 2 IBr(g) = 12(g) + Br2(g) Suppose we
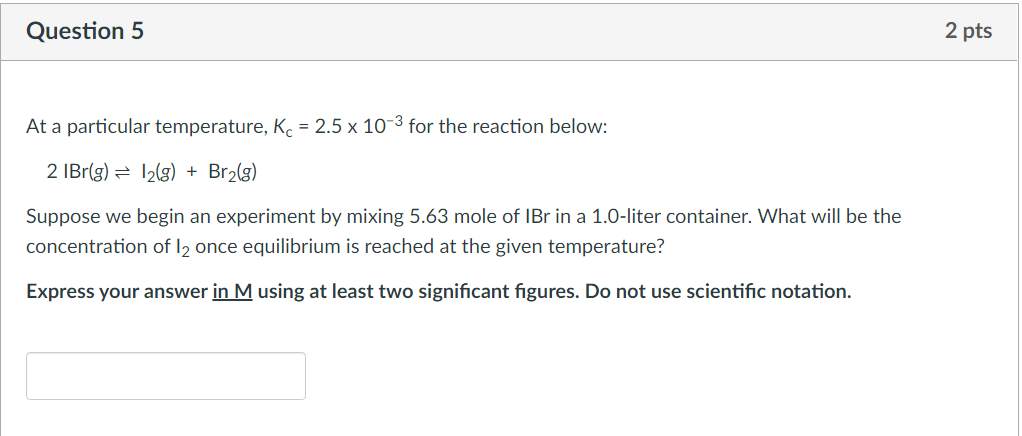
Question 5 2 pts At a particular temperature, Kc = 2.5 x 10-3 for the reaction below: 2 IBr(g) = 12(g) + Br2(g) Suppose we begin an experiment by mixing 5.63 mole of Br in a 1.0-liter container. What will be the concentration of 12 once equilibrium is reached at the given temperature? Express your answer in M using at least two significant figures. Do not use scientific notation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


