Question: Question 5 2 pts During lab, a student used a Mohr pipet to add the following solutions into a 25 mL volumetric flask. They calculated
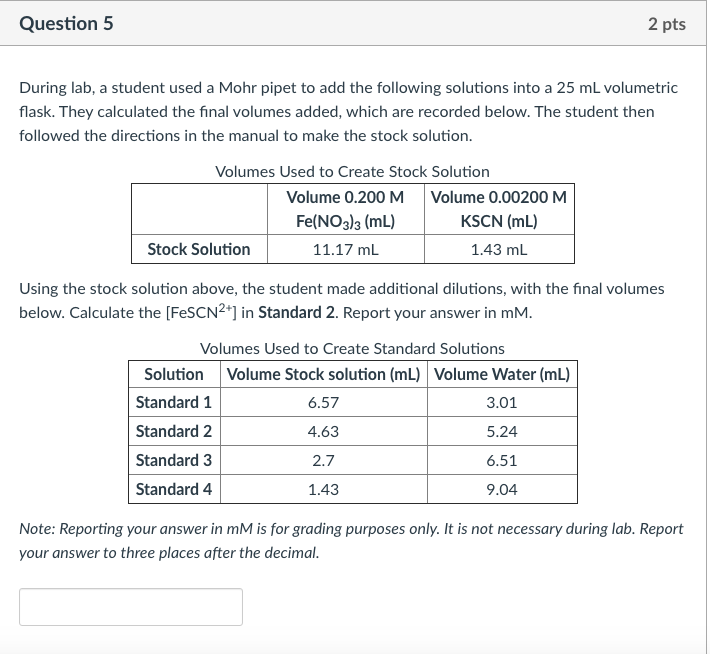
Question 5 2 pts During lab, a student used a Mohr pipet to add the following solutions into a 25 mL volumetric flask. They calculated the final volumes added, which are recorded below. The student then followed the directions in the manual to make the stock solution. Volumes Used to Create Stock Solution Volume 0.200 M Volume 0.00200 M Fe(NO3)3 (mL) KSCN (mL) Stock Solution 11.17 ml 1.43 mL Using the stock solution above, the student made additional dilutions, with the final volumes below. Calculate the [FeSCN2+] in Standard 2. Report your answer in mM. Volumes Used to Create Standard Solutions Solution Volume Stock solution (mL) Volume Water (mL) Standard 1 6.57 3.01 Standard 2 4.63 5.24 Standard 3 2.7 6.51 Standard 4 1.43 9.04 Note: Reporting your answer in mM is for grading purposes only. It is not necessary during lab. Report your answer to three places after the decimal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


