Question: Question 5 5 Points To study the Gibbs adsorption isotherm by capillary rise method, different concentrations of butanol and NaCl were prepared at 20. Following
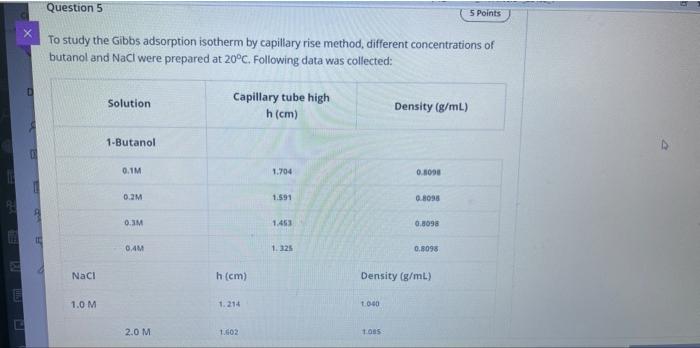
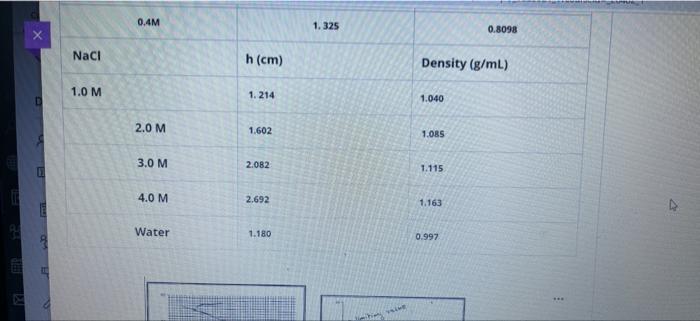
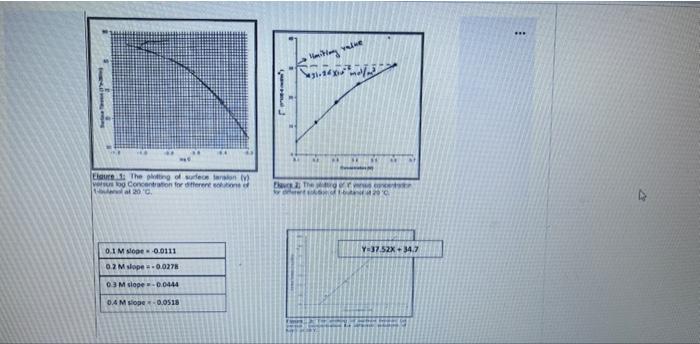
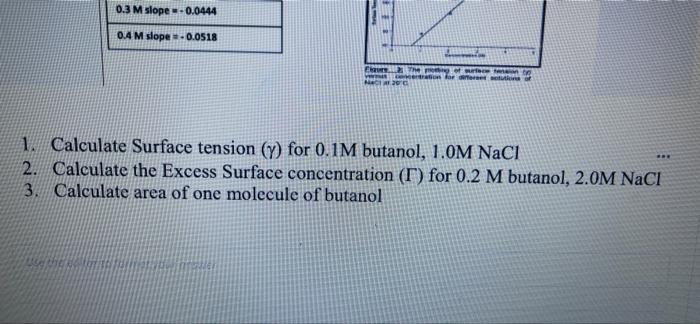
Question 5 5 Points To study the Gibbs adsorption isotherm by capillary rise method, different concentrations of butanol and NaCl were prepared at 20. Following data was collected: D Solution Capillary tube high h(cm) Density (g/mL) 1-Butanol 0.1M 1.704 0.5000 0.2M 1.591 0.8098 0.3M 1.463 0.0098 0.41 1.325 0.8098 Naci h (cm) Density (g/ml) 1.0 M 1.214 1040 2.0 M 1.602 tos 0.4M 1. 325 0.8098 Naci h(cm) Density (g/mL) 1.0 M 1.214 1.040 2.0 M 1.602 1.085 3.0 M 2.082 1.115 4.0 M 2.692 1.163 E Water 1.180 0.997 29. lue Vittory 1- ** Elure The Dog of surferson O Concentration et 20 Rhe 420 Y=37.52X +34.7 01 M slope -0.0111 02 M slope = -0.0278 03 M slope -0.0444 04 M slope -0.0518 0.3 M slope -0.0444 0.4 M slope. 0.0518 E The LE Go for retete MO 1. Calculate Surface tension (Y) for 0.1M butanol, 1.0M NaCl 2. Calculate the Excess Surface concentration (T) for 0.2 M butanol, 2.0M NaCl 3. Calculate area of one molecule of butanol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


