Question: QUESTION 5 (8 marks) In the early 1900s, scientists modelled the hydrogen atom as an electron orbiting a proton. Like when we look at the
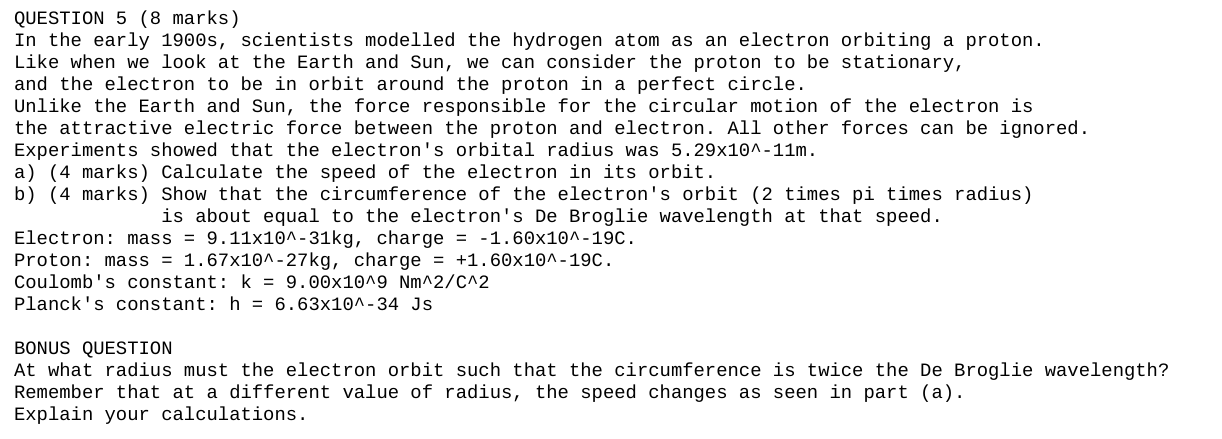
QUESTION 5 (8 marks) In the early 1900s, scientists modelled the hydrogen atom as an electron orbiting a proton. Like when we look at the Earth and Sun, we can consider the proton to be stationary, and the electron to be in orbit around the proton in a perfect circle. Unlike the Earth and Sun, the force responsible for the circular motion of the electron is the attractive electric force between the proton and electron. All other forces can be ignored. Experiments showed that the electron's orbital radius was 5. 29x10^-11m. a) (4 marks) Calculate the speed of the electron in its orbit. b) (4 marks) Show that the circumference of the electron's orbit (2 times pi times radius) is about equal to the electron's De Broglie wavelength at that speed. Electron: mass = 9. 11x10^-31kg, charge = -1. 60x10^-190. Proton: mass = 1. 67x10^-27kg, charge = +1. 60x10^-190. Coulomb's constant: k = 9. 00x10^9 Nm^2/C^2 Planck's constant: h = 6. 63x10^-34 Js BONUS QUESTION At what radius must the electron orbit such that the circumference is twice the De Broglie wavelength? Remember that at a different value of radius, the speed changes as seen in part (a) . Explain your calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


