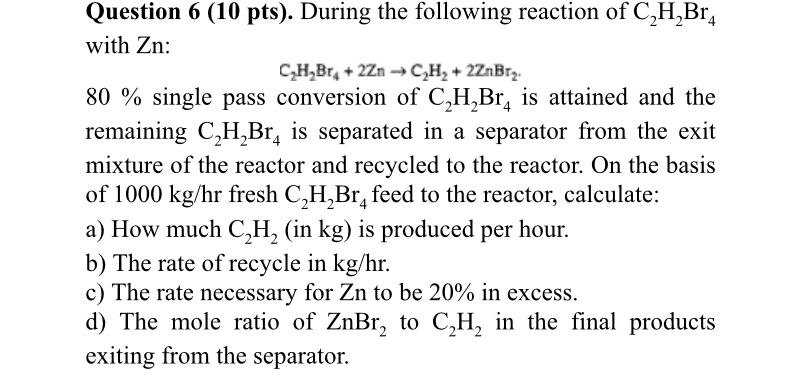
Question: Question 6 ( 1 0 pts ) . During the following reaction of C 2 H 2 B r 4 with Z n : C
Question pts During the following reaction of with :
single pass conversion of is attained and the remaining is separated in a separator from the exit mixture of the reactor and recycled to the reactor. On the basis of fresh feed to the reactor, calculate:
a How much in is produced per hour.
b The rate of recycle in
c The rate necessary for to be in excess.
d The mole ratio of to in the final products exiting from the separator.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


