Question: Question 6. Under normal atmospheric pressure (1 atm), a stream of air (at 2C and the percentage relative humidity of 15) enters a moisturizer shown
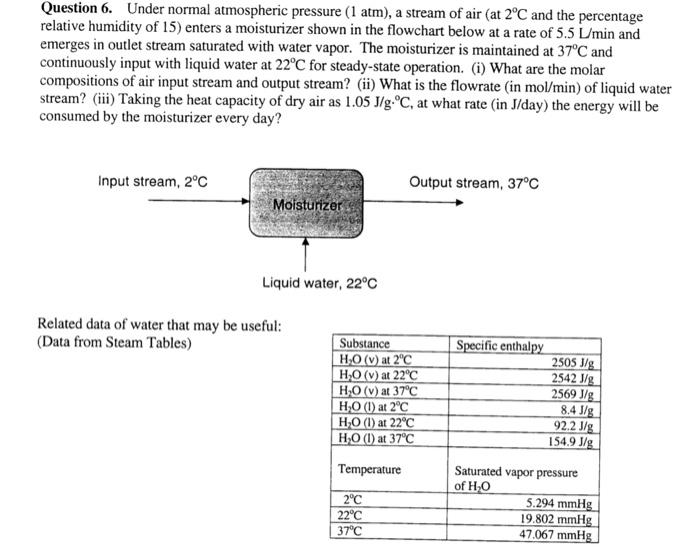
Question 6. Under normal atmospheric pressure ( 1atm ), a stream of air (at 2C and the percentage relative humidity of 15 ) enters a moisturizer shown in the flowchart below at a rate of 5.5L/min and emerges in outlet stream saturated with water vapor. The moisturizer is maintained at 37C and continuously input with liquid water at 22C for steady-state operation. (i) What are the molar compositions of air input stream and output stream? (ii) What is the flowrate (in mol/min ) of liquid water stream? (iii) Taking the heat capacity of dry air as 1.05J/g.C, at what rate (in J/ day) the energy will be consumed by the moisturizer every day? Related data of water that may be useful: (Data from Steam Tables)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


