Question: Question 7 : ( 2 0 % ) Liquid water at ( 1 5 ^ { circ } mathrm { C
Question :
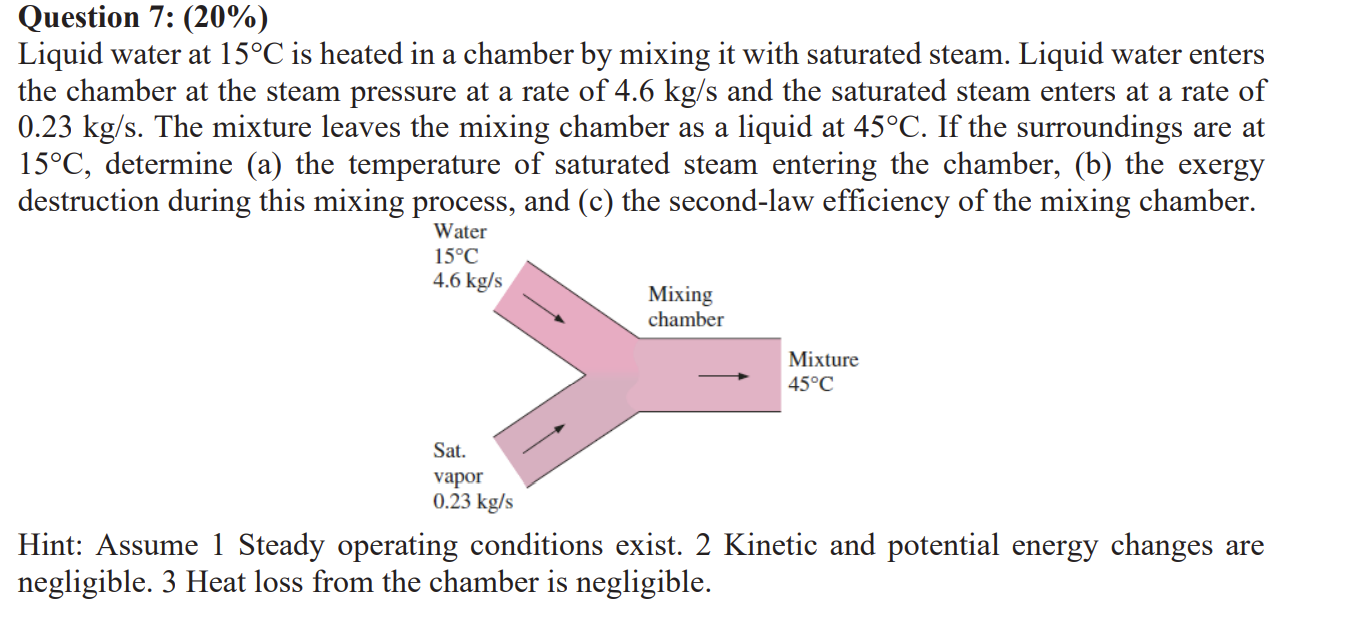
Liquid water at circmathrmC is heated in a chamber by mixing it with saturated steam. Liquid water enters the chamber at the steam pressure at a rate of mathrm~kgmathrms and the saturated steam enters at a rate of mathrm~kgmathrms The mixture leaves the mixing chamber as a liquid at circmathrmC If the surroundings are at circmathrmC determine a the temperature of saturated steam entering the chamber, b the exergy destruction during this mixing process, and c the secondlaw efficiency of the mixing chamber.
Hint: Assume Steady operating conditions exist. Kinetic and potential energy changes are negligible. Heat loss from the chamber is negligible.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


