Question: Question 7: ( 3 points) A 500mL solution containing 1.17gNaCl is mixed with a 500mL solution containing 1.49gKCl. What is the final ppm concentration of
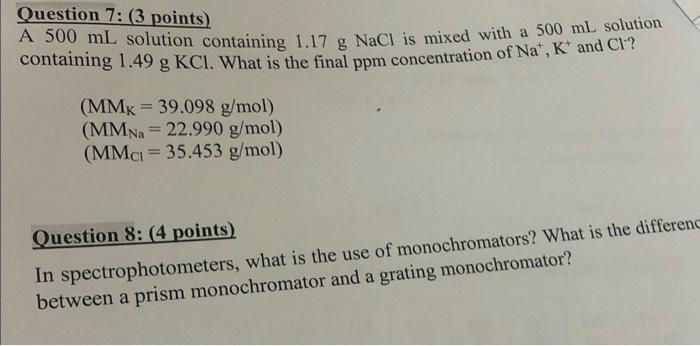
Question 7: ( 3 points) A 500mL solution containing 1.17gNaCl is mixed with a 500mL solution containing 1.49gKCl. What is the final ppm concentration of Na+,K+and Cl? (MMK=39.098g/mol)(MMNa=22.990g/mol)(MMCl=35.453g/mol) Question 8: (4 points) In spectrophotometers, what is the use of monochromators? What is the differen between a prism monochromator and a grating monochromator
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


