Question: QUESTION 7 6 points Save Answer A solution is prepared such that the initial concentration of A2+ is 1.50x10-3 M and the initial concentration of
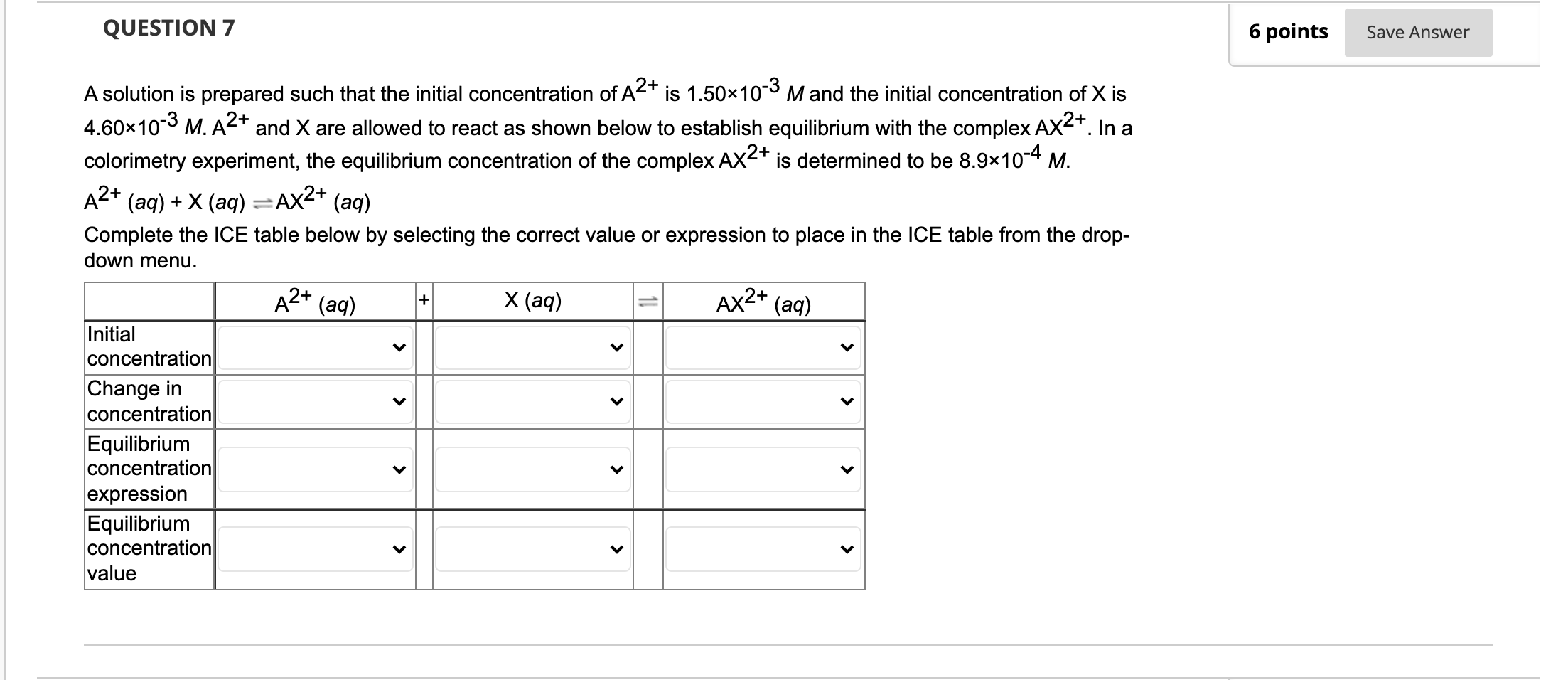
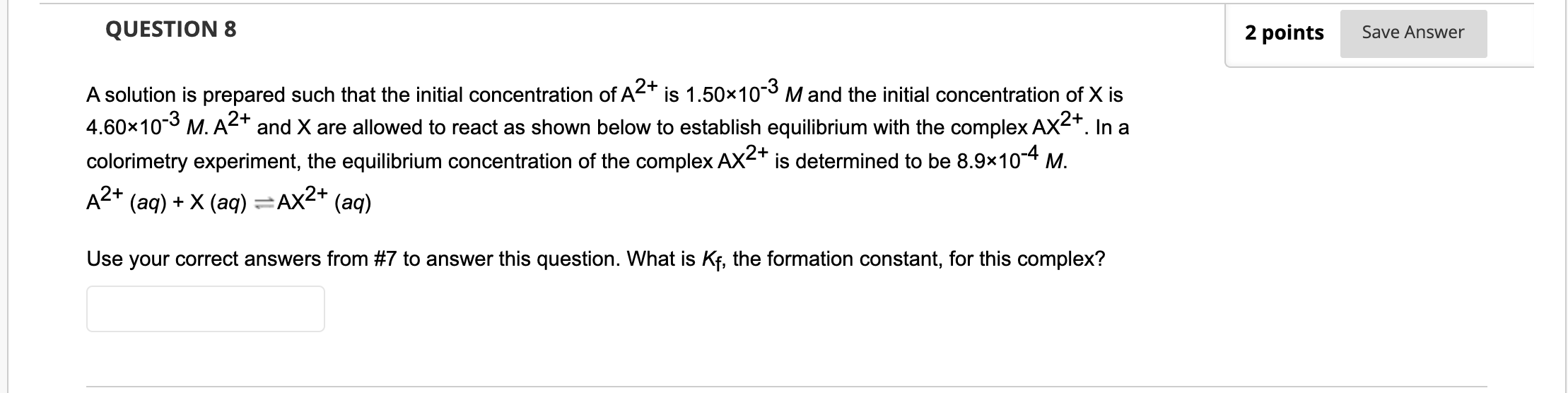
QUESTION 7 6 points Save Answer A solution is prepared such that the initial concentration of A2+ is 1.50x10-3 M and the initial concentration of X is 4.60*10-3 M. A2+ and X are allowed to react as shown below to establish equilibrium with the complex AX2+. In a colorimetry experiment, the equilibrium concentration of the complex AX2+ is determined to be 8.9x10-4 M. (aq) + X (aq) =AX2+ (aq) Complete the ICE table below by selecting the correct value or expression to place in the ICE table from the drop- down menu. A2+ A2+ (aq) X (aq) AX2+ (aq) Initial concentration Change in concentration Equilibrium concentration expression Equilibrium concentration value
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


