Question: Question 7 CC To prepare a buffer solution with pH = 2.6, using one of the following weak acids: HA (Ka=2.7x10-3), HB (Ka-4.4x10-6), HC (Ka=2.6x10-9
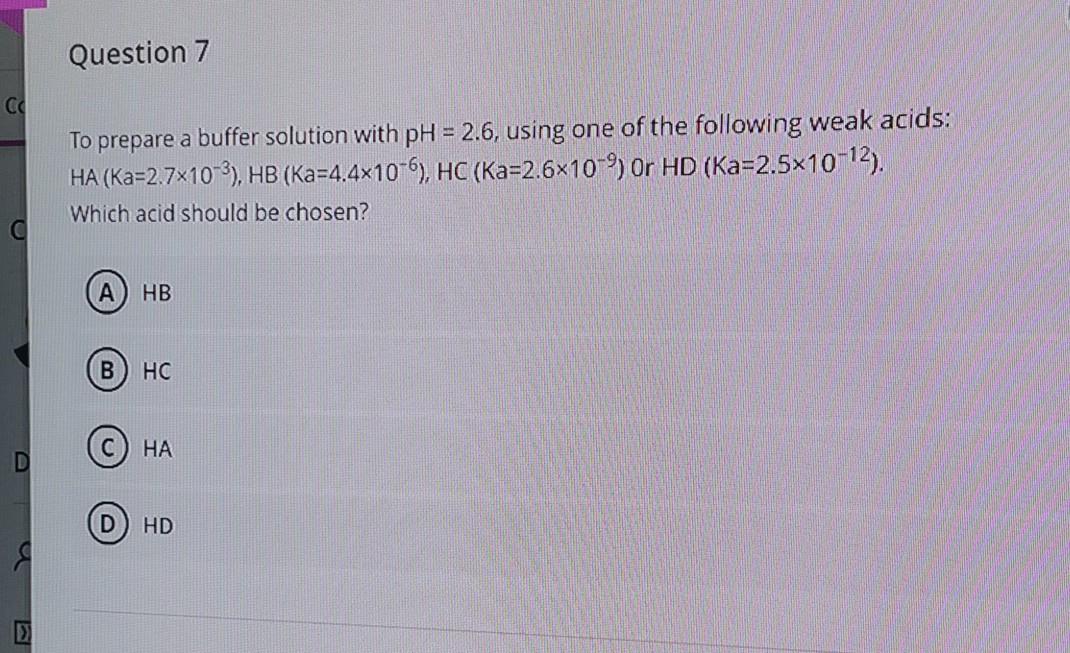
Question 7 CC To prepare a buffer solution with pH = 2.6, using one of the following weak acids: HA (Ka=2.7x10-3), HB (Ka-4.4x10-6), HC (Ka=2.6x10-9 or HD (Ka=2.5x10-12). Which acid should be chosen? c A HB B HC HA D D HD
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


