Question: QUESTION 7 How much energy, in k J , is required to heat a 1 2 5 g sample of benzene ) = ( 7
QUESTION
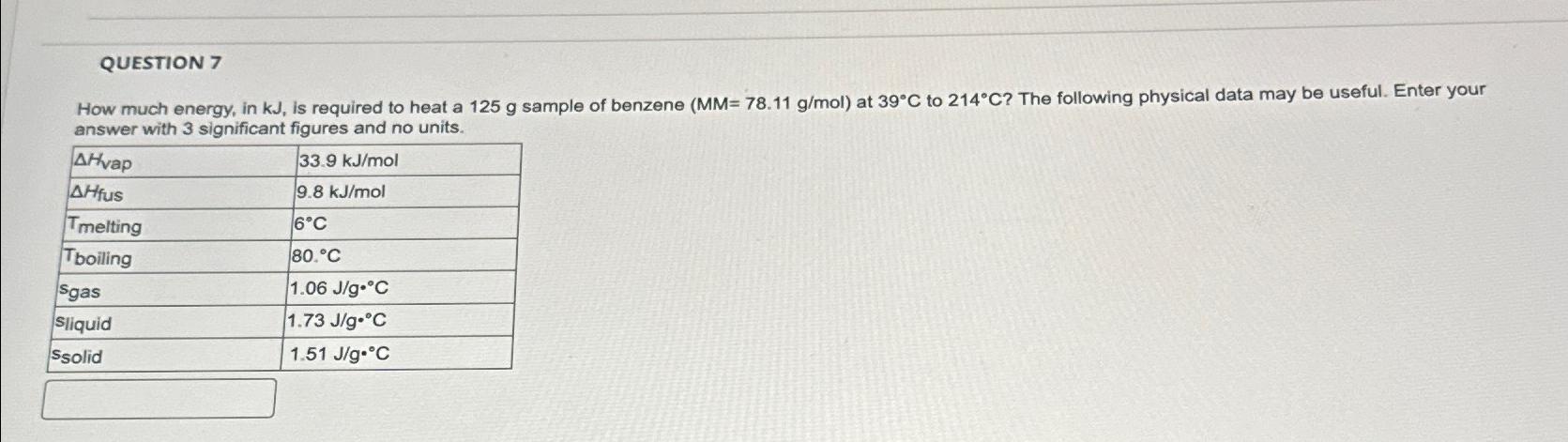
How much energy, in is required to heat a sample of benzene at to The following physical data may be useful. Enter your answer with significant figures and no units.
table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


