Question: Question 8 6 pts The formation constant for the complex formed between Ni2and EDTA is 3.6 x 1018 A 25.00 mL aliquot of 0.300 M
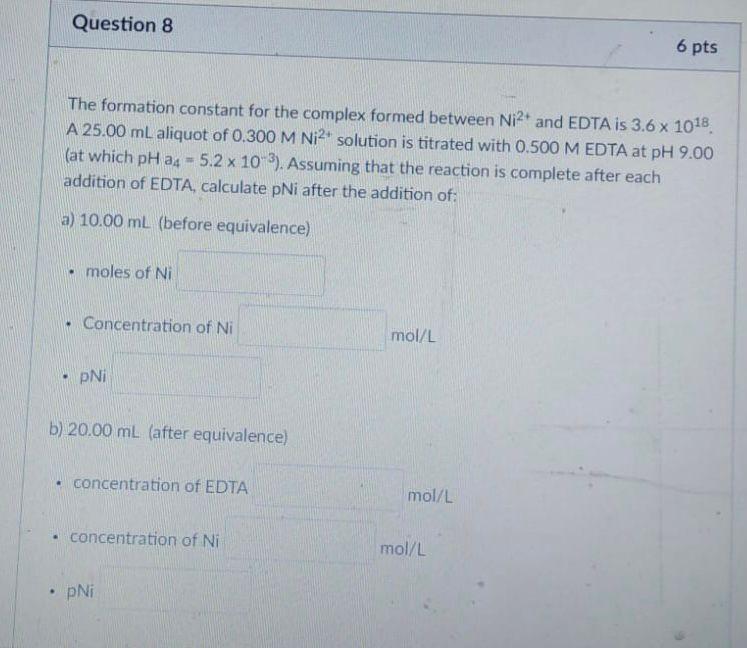
Question 8 6 pts The formation constant for the complex formed between Ni2and EDTA is 3.6 x 1018 A 25.00 mL aliquot of 0.300 M Ni2+ solution is titrated with 0.500 M EDTA at pH 9.00 (at which pH a4 = 5.2 x 10-3). Assuming that the reaction is complete after each addition of EDTA, calculate pNi after the addition of: a) 10.00 mL (before equivalence) moles of Ni Concentration of Ni mol/L PNI b) 20.00 mL (after equivalence) . concentration of EDTA mol/L concentration of Ni mol/L . pNi
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


