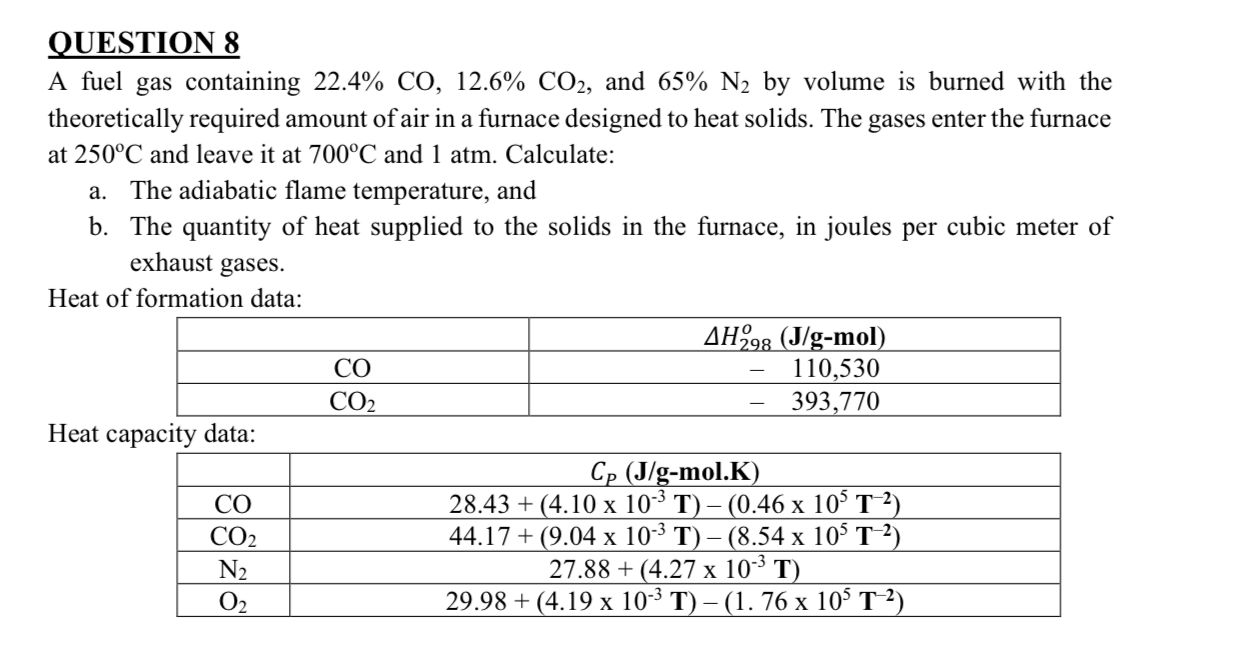
Question: QUESTION 8 A fuel gas containing 2 2 . 4 % C O , 1 2 . 6 % C O 2 , and 6
QUESTION
A fuel gas containing and by volume is burned with the
theoretically required amount of air in a furnace designed to heat solids. The gases enter the furnace
at and leave it at and atm. Calculate:
a The adiabatic flame temperature, and
b The quantity of heat supplied to the solids in the furnace, in joules per cubic meter of
exhaust gases.
Heat of formation data:
Heat capacity data:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


