Question: Question 8 A solution with PH of 9.7 has a [H*) of (Assume 25C, Kw=1x10-14) A. 6.80 x 10'M B. 2.00 x 10-10 M C.
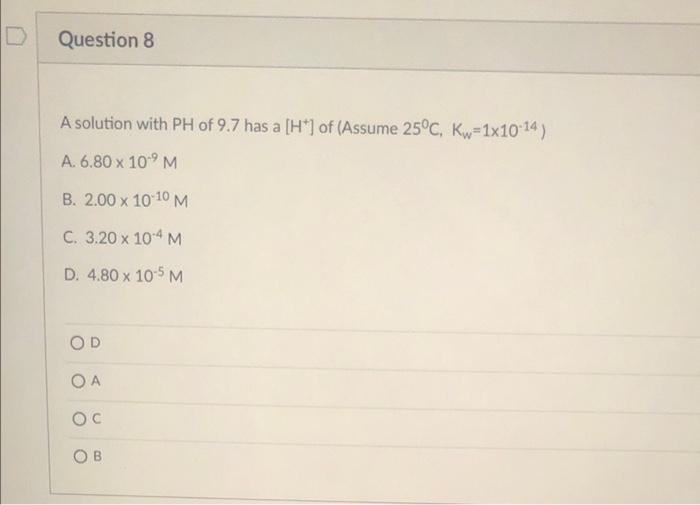
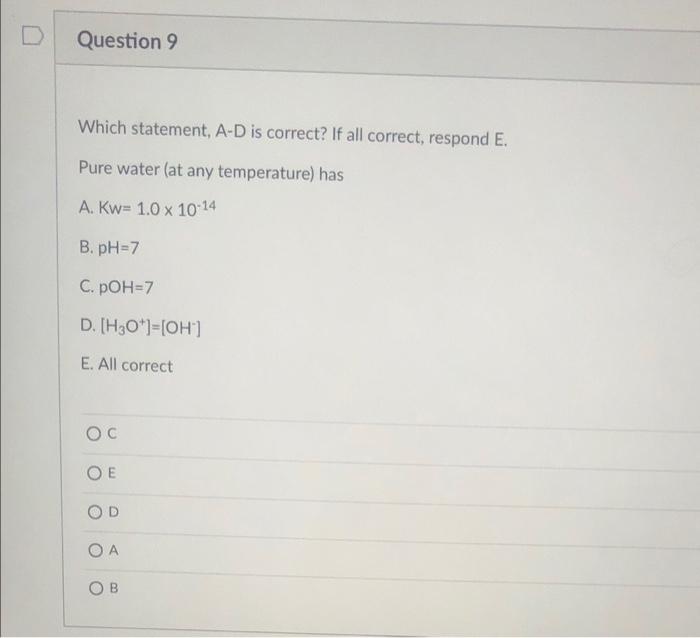
Question 8 A solution with PH of 9.7 has a [H*) of (Assume 25C, Kw=1x10-14) A. 6.80 x 10'M B. 2.00 x 10-10 M C. 3.20 x 10-4 M D. 4.80 x 10-5 M X D Question 9 Which statement, A-D is correct? If all correct, respond E. Pure water (at any temperature) has A. Kw= 1.0 x 10-14 B. pH=7 C. POH=7 D. [H3O*)=[OH') E. All correct OD
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


