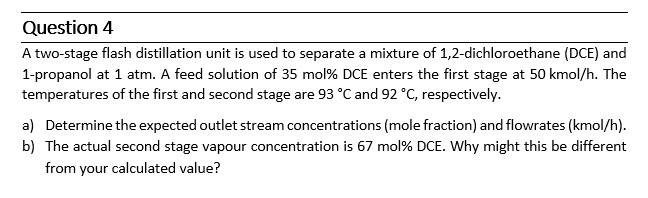
Question: Question 8 A two - stage flash distillation unit is used to separate a mixture of 1 , 2 - dichloroethane ( DCE ) and
Question
A twostage flash distillation unit is used to separate a mixture of dichloroethane DCE and propanol at atm. A feed solution of mol DCE enters the first stage at kmo The
temperatures of the first and second stage are and respectively.
a Determine the expected outlet stream concentrations mole fraction and flowrates kmo
b The actual second stage vapour concentration is mol DCE. Why might this be different
from your calculated value?A twostage flash distillation unit is used to separate a mixture of dichloroethane DCE and propanol at atm A feed solution of mol DCE enters the first stage at kmol h The temperatures of the first and second stage are C and C respectively.
a Determine the expected outlet stream concentrations mole fraction and flowrates kmolh
b The actual second stage vapour concentration is mol DCE. Why might this be different from your calculated value?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


