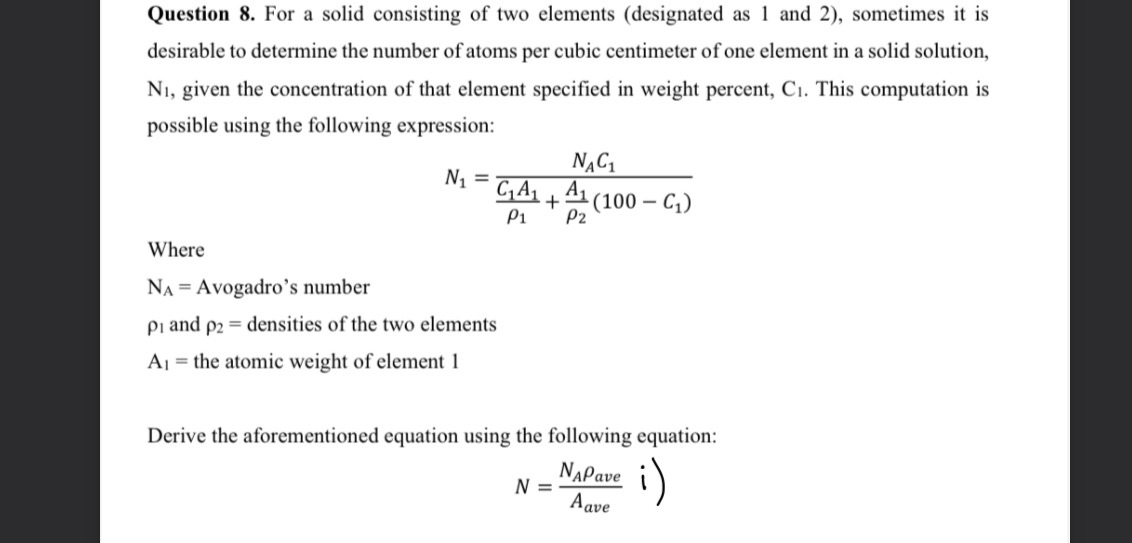
Question: Question 8 . For a solid consisting of two elements ( designated as 1 and 2 ) , sometimes it is desirable to determine the
Question For a solid consisting of two elements designated as and sometimes it is
desirable to determine the number of atoms per cubic centimeter of one element in a solid solution,
given the concentration of that element specified in weight percent, This computation is
possible using the following expression:
Where
Avogadro's number
and densities of the two elements
the atomic weight of element
Derive the aforementioned equation using the following equation:
:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


