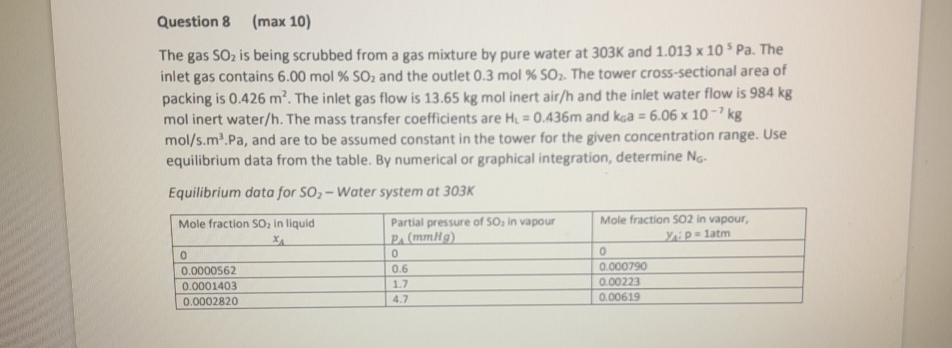
Question: Question 8 ( m a x 1 0 ) The gas S O 2 is being scrubbed from a gas mixture by pure water at
Question
The gas is being scrubbed from a gas mixture by pure water at and The inlet gas contains mol and the outlet mol The tower crosssectional area of packing is The inlet gas flow is kgmol inert airh and the inlet water flow is mol inert water The mass transfer coefficients are and and are to be assumed constant in the tower for the given concentration range. Use equilibrium data from the table. By numerical or graphical integration, determine
Equilibrium data for Water system at
tabletableMole fraction in liquid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


